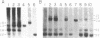Abstract
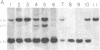
Previous theoretical considerations and some experimental data have suggested a role for DNA methylation in the maintenance of mammalian X chromosome inactivation. The isolation of specific X-encoded sequences makes it possible to investigate this hypothesis directly. We have used cloned fragments of the human hypoxanthine phosphoribosyltransferase (HPRT) gene and methylation-sensitive restriction enzymes to study methylation patterns in genomic DNA of individuals with different numbers of X chromosomes and in somatic cell hybrid lines containing human X chromosomes that are either active or inactive or have been reactivated by treatment with 5-azacytidine. The results of these analyses show that there is hypomethylation of active X chromosomes relative to inactive X chromosomes in the 5' region of this gene. In the middle region of the gene, however, a site that is consistently undermethylated on inactive X chromosomes was identified. Taken together, the data suggest that the overall pattern of methylation, rather than methylation of specific sites, plays a role in the maintenance of X chromosome inactivation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORENFREUND E., FITT E., BENDICH A. Isolation and properties of deoxyribonucleic acid from mammalian sperm. Nature. 1961 Sep 30;191:1375–1377. doi: 10.1038/1911375a0. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman V. M., Kratzer P. G., Siracusa L. D., Quarantillo B. A., Evans R., Liskay R. M. Evidence for DNA modification in the maintenance of X-chromosome inactivation of adult mouse tissues. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5357–5361. doi: 10.1073/pnas.79.17.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinault A. C., Carbon J. Overlap hybridization screening: isolation and characterization of overlapping DNA fragments surrounding the leu2 gene on yeast chromosome III. Gene. 1979 Feb;5(2):111–126. doi: 10.1016/0378-1119(79)90097-0. [DOI] [PubMed] [Google Scholar]
- Erickson R. P., Kramer J. M., Rittenhouse J., Salkeld A. Quantitation of mRNAs during mouse spermatogenesis: protamine-like histone and phosphoglycerate kinase-2 mRNAs increase after meiosis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6086–6090. doi: 10.1073/pnas.77.10.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler S. M., Andina R. J. Mammalian X-chromosome inactivation. Adv Hum Genet. 1976;7:99–140. doi: 10.1007/978-1-4757-0659-8_3. [DOI] [PubMed] [Google Scholar]
- Geever R. F., Wilson L. B., Nallaseth F. S., Milner P. F., Bittner M., Wilson J. T. Direct identification of sickle cell anemia by blot hybridization. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5081–5085. doi: 10.1073/pnas.78.8.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Mammalian gonadal determination and gametogenesis. Science. 1981 Mar 20;211(4488):1265–1271. doi: 10.1126/science.6259727. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer P. G., Chapman V. M., Lambert H., Evans R. E., Liskay R. M. Differences in the DNA of the inactive X chromosomes of fetal and extraembryonic tissues of mice. Cell. 1983 May;33(1):37–42. doi: 10.1016/0092-8674(83)90332-x. [DOI] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Lifschytz E., Lindsley D. L. The role of X-chromosome inactivation during spermatogenesis (Drosophila-allocycly-chromosome evolution-male sterility-dosage compensation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):182–186. doi: 10.1073/pnas.69.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M. The effect of sequence specific DNA methylation on restriction endonuclease cleavage. Nucleic Acids Res. 1981 Nov 25;9(22):5859–5866. doi: 10.1093/nar/9.22.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon C., Ohkubo H., Pastan I., de Crombrugghe B. Unusual methylation pattern of the alpha 2 (l) collagen gene. Cell. 1982 May;29(1):203–210. doi: 10.1016/0092-8674(82)90104-0. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Okamoto E., Erlanger B. F., Miller O. J. Is DNA methylation responsible for mammalian X chromosome inactivation? Cytogenet Cell Genet. 1982;33(4):345–349. doi: 10.1159/000131782. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Shapiro L. J., Sparkes R. S., Sparkes M. C. Regional assignment of the steroid sulfatase-X-linked ichthyosis locus: implications for a noninactivated region on the short arm of human X chromosome. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5779–5783. doi: 10.1073/pnas.76.11.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Hellkuhl B., Grzeschik K. H., Shapiro L. J. Expression of an X-linked gene from an inactive human X chromosome in mouse-human hybrid cells: further evidence for the noninactivation of the steroid sulfatase locus in man. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6759–6763. doi: 10.1073/pnas.77.11.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Genetic evidence for the inactivation of a human autosomal locus attached to an inactive X chromosome. Am J Hum Genet. 1982 Sep;34(5):811–817. [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Hereford L. Identification of a sequence responsible for periodic synthesis of yeast histone 2A mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7689–7693. doi: 10.1073/pnas.79.24.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Santi D. V., Garrett C. E., Barr P. J. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell. 1983 May;33(1):9–10. doi: 10.1016/0092-8674(83)90327-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M. Transformation of Hprt gene with sperm DNA. Somatic Cell Genet. 1983 Sep;9(5):617–627. doi: 10.1007/BF01574262. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Studies of X chromosome DNA methylation in normal human cells. Nature. 1982 Feb 25;295(5851):667–671. doi: 10.1038/295667a0. [DOI] [PubMed] [Google Scholar]