Abstract
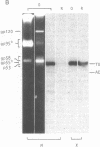
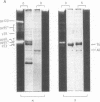
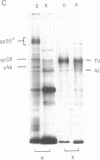
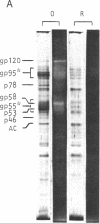
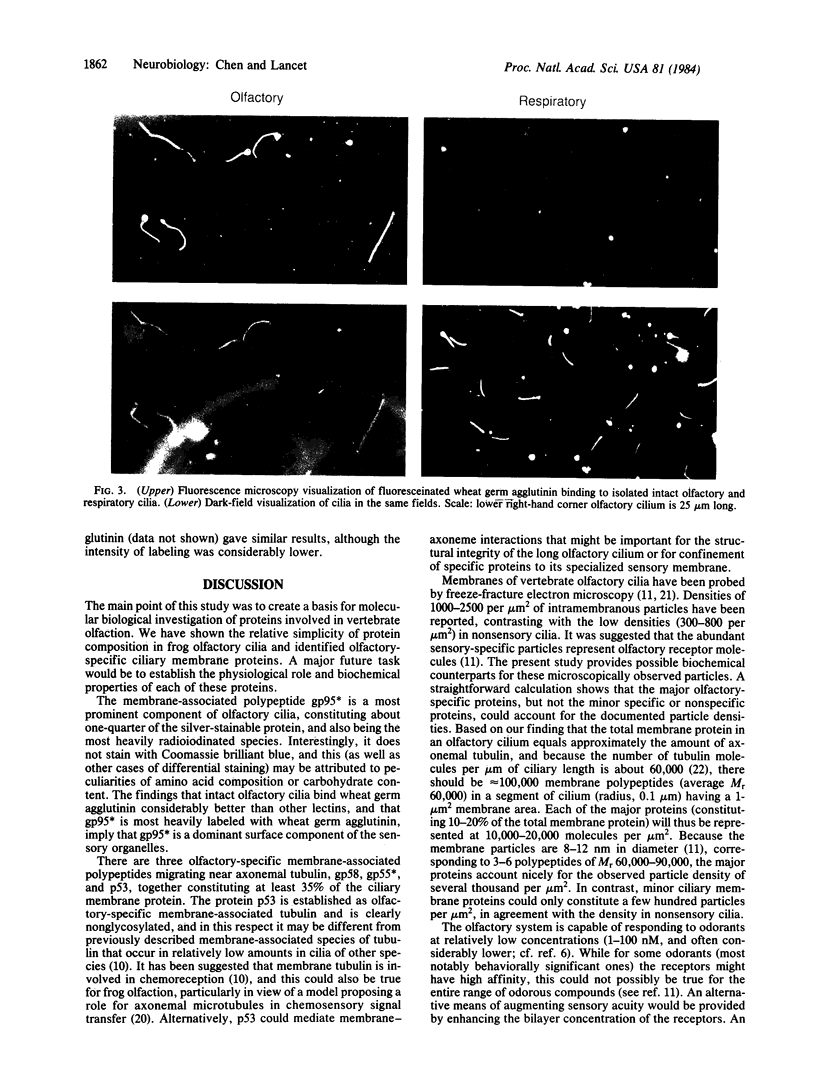
In search for olfactory receptor molecules, we carried out comprehensive electrophoretic mapping of membrane proteins in the cilia of frog olfactory epithelium. Seven polypeptides, extracted from isolated cilia by nonionic detergent, were unique to the sensory organelles, compared to nonsensory (respiratory) counterparts. Olfactory cilia contained 3-10 times more membrane-associated protein as compared to respiratory cilia, in agreement with reported densities of freeze-fracture intramembranous particles. Four of the olfactory polypeptides were major constituents of the ciliary membrane, each amounting to greater than 10% of its total protein. Three major and one minor specific polypeptide were glycosylated, whereas membranes of nonsensory cilia were practically devoid of glycoproteins. A clear difference in surface composition was also shown by microscopic visualization of fluoresceinated lectin bound to intact isolated cilia. Two of the olfactory glycoproteins displayed pronounced heterogeneity of apparent molecular weight, which could partly be due to protein sequence diversity, as expected for odorant receptor molecules. The recently described inhibition of odorant-evoked sensory potentials by the lectin concanavalin A is consistent with the hypothesis that one or more of the specific glycoproteins described here plays a role in olfactory reception.
Full text
PDF
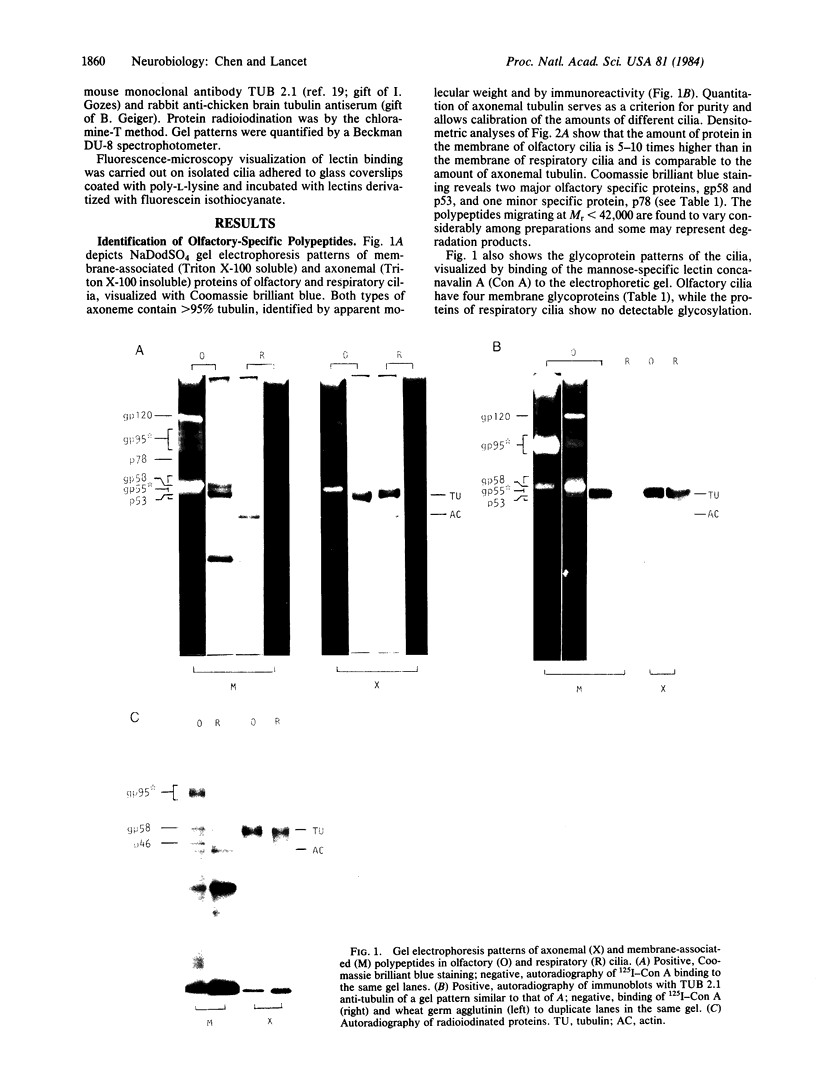
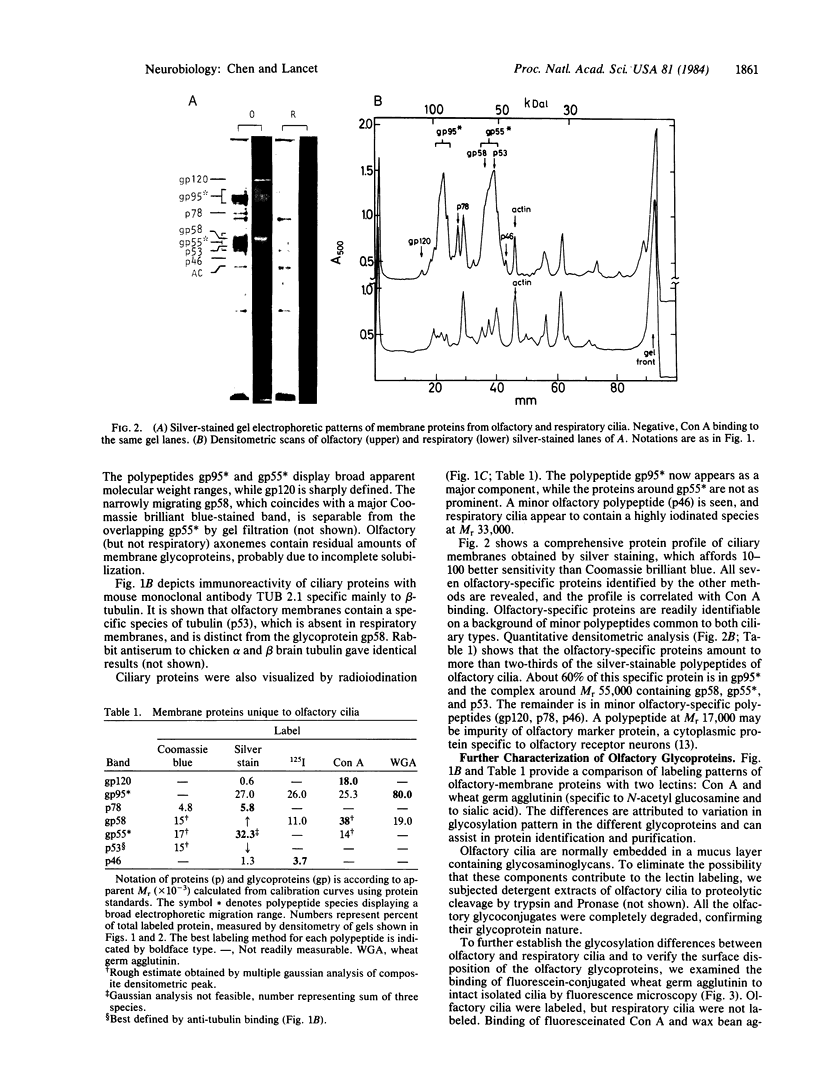

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atema J. Microtube theory of sensory transduction. J Theor Biol. 1973 Jan;38(1):181–190. doi: 10.1016/0022-5193(73)90233-6. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Beauchamp G. K., Yamazaki K., Bard J., Thomas L. Chemosensory communication. A new aspect of the major histocompatibility complex and other genes in the mouse. Oncodev Biol Med. 1982;4(1-2):101–116. [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Getchell T. V. Unitary responses in frog olfactory epithelium to sterically related molecules at low concentrations. J Gen Physiol. 1974 Aug;64(2):241–261. [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Barnstable C. J. Monoclonal antibodies that recognize discrete forms of tubulin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2579–2583. doi: 10.1073/pnas.79.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., Margolis F. L. Isolation and characterization of rat olfactory marker protein. J Biol Chem. 1976 Oct 25;251(20):6232–6237. [PubMed] [Google Scholar]
- Kerjaschki D., Hörander H. The development of mouse olfactory vesicles and their cell contacts: a freeze-etching study. J Ultrastruct Res. 1976 Mar;54(3):420–444. doi: 10.1016/s0022-5320(76)80027-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linck R. W. Comparative isolation of cilia and flagella from the lamellibranch mollusc, Aequipecten irradians. J Cell Sci. 1973 Mar;12(2):345–367. doi: 10.1242/jcs.12.2.345. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Menco B. P. Qualitative and quantitative freeze-fracture studies on olfactory and nasal respiratory epithelial surfaces of frog, ox, rat, and dog. II. Cell apices, cilia, and microvilli. Cell Tissue Res. 1980;211(1):5–29. doi: 10.1007/BF00233719. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- O'Brien D. F. The chemistry of vision. Science. 1982 Dec 3;218(4576):961–966. doi: 10.1126/science.6291153. [DOI] [PubMed] [Google Scholar]
- Rhein L. D., Cagan R. H. Biochemical studies of olfaction: isolation, characterization, and odorant binding activity of cilia from rainbow trout olfactory rosettes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4412–4416. doi: 10.1073/pnas.77.8.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. E. Major membrane protein differences in cilia and flagella: evidence for a membrane-associated tubulin. Biochemistry. 1977 May 17;16(10):2047–2058. doi: 10.1021/bi00629a001. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]