Abstract
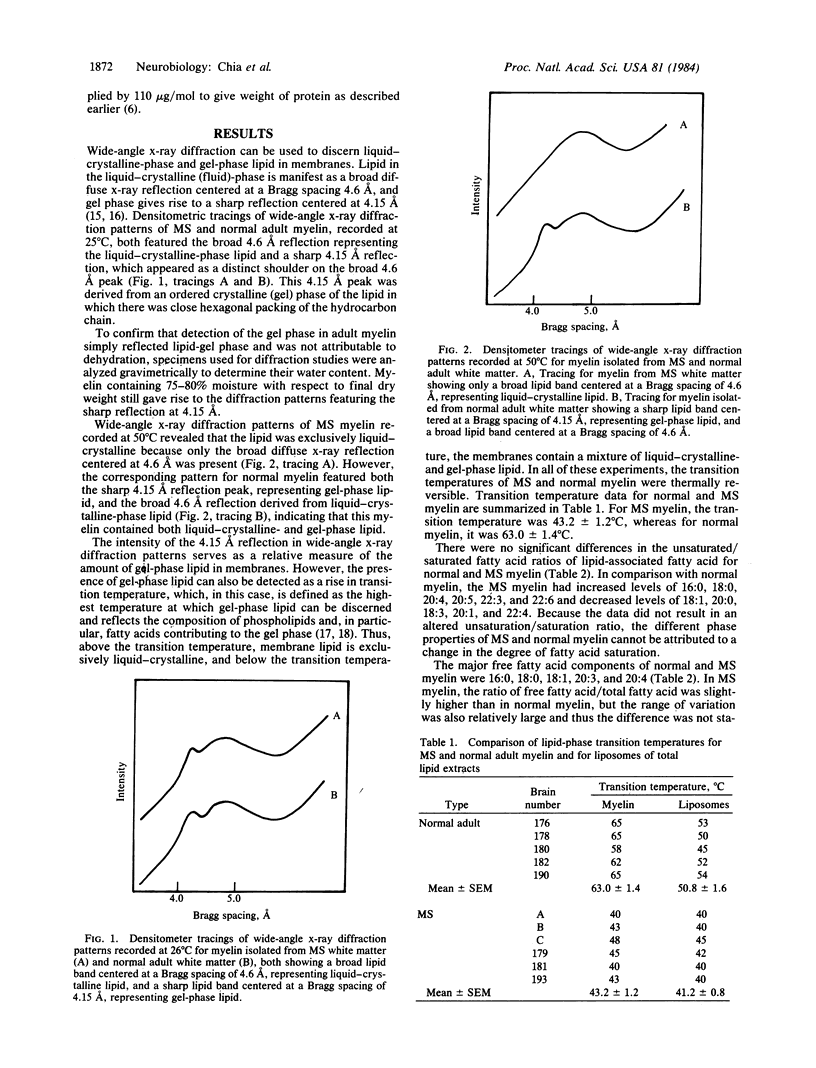
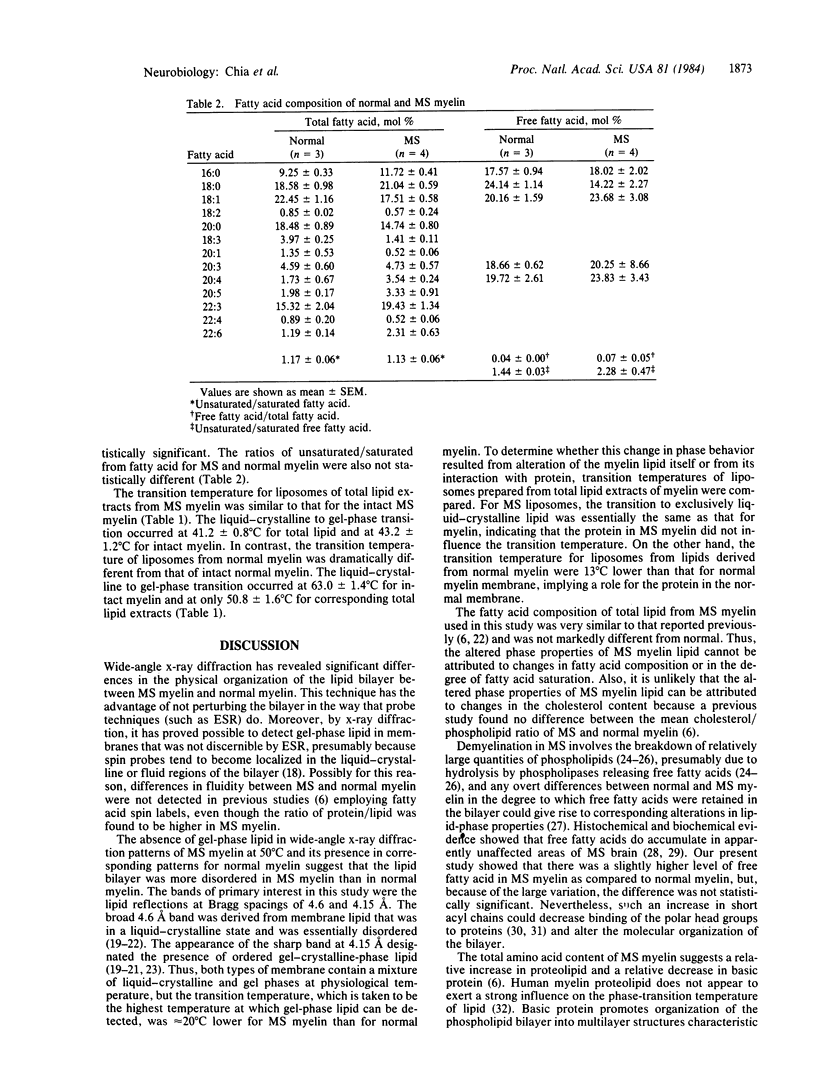
Wide-angle x-ray diffraction studies revealed that the lipid-phase transition temperature of multiple sclerosis (MS) myelin was about 20 degrees C lower than that of normal myelin, indicating differences in the physical organization of the bilayer. The transition temperature of liposomes prepared from total lipid extracts of normal myelin was 12 degrees C lower than that for corresponding intact myelin, demonstrating that the protein of normal myelin had a substantial ordering effect on the lipid bilayer. The transition temperature for liposomes of MS myelin lipid was essentially similar to that for isolated MS myelin. Because the protein/phospholipid ratio was higher in MS myelin, and no difference in degree of fatty acid saturation was observed, the inability of MS myelin protein to organize the lipid reflects a qualitative difference in the proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS C. W., TUQAN N. A. Histochemistry of myelin. II. Proteins, lipid-protein dissociation and proteinase activity in Wallerian degeneration. J Neurochem. 1961 Mar;6:334–341. doi: 10.1111/j.1471-4159.1961.tb13484.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Boggs J. M., Clement I. R., Moscarello M. A. Similar effect of proteolipid apoproteins from human myelin (lipophilin) and bovine white matter on the lipid phase transition. Biochim Biophys Acta. 1980 Sep 2;601(1):134–151. doi: 10.1016/0005-2736(80)90520-9. [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Moscarello M. A. A comparison of composition and fluidity of multiple sclerosis and normal myelin. Neurochem Res. 1980 Mar;5(3):319–336. doi: 10.1007/BF00964620. [DOI] [PubMed] [Google Scholar]
- Brady G. W., Fein D. B., Wood D. D., Moscarello M. A. The interaction of basic proteins from normal and multiple sclerosis myelin with phosphatidylglycerol vesicles. FEBS Lett. 1981 Mar 23;125(2):159–160. doi: 10.1016/0014-5793(81)80708-9. [DOI] [PubMed] [Google Scholar]
- Brady G. W., Murthy N. S., Fein D. B., Wood D. D., Moscarello M. A. The effect of basic myelin protein on multilayer membrane formation. Biophys J. 1981 May;34(2):345–350. doi: 10.1016/S0006-3495(81)84853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia L. S., Thompson J. E., Dumbroff E. B. Simulation of the effects of leaf senescence on membranes by treatment with paraquat. Plant Physiol. 1981 Mar;67(3):415–420. doi: 10.1104/pp.67.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia L. S., Thompson J. E., Moscarello M. A. Changes in lipid phase behaviour in human myelin during maturation and aging. Involvement of lipid peroxidation. FEBS Lett. 1983 Jun 27;157(1):155–158. doi: 10.1016/0014-5793(83)81136-3. [DOI] [PubMed] [Google Scholar]
- Davison A. N. Chemical pathology of brain degeneration. Proc R Soc Med. 1977 May;70(5):349–351. doi: 10.1177/003591577707000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M. Lipid bilayer structure in the membrane of Mycoplasma laidlawii. J Mol Biol. 1971 May 28;58(1):153–165. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. X-ray diffraction studies of phase transitions in the membrane of Mycoplasma laidlawii. J Mol Biol. 1970 Jan 14;47(1):115–117. doi: 10.1016/0022-2836(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoyama Y., Sternberger N. H., Webster H. D., Quarles R. H., Cohen S. R., Richardson E. P., Jr Immunocytochemical observations on the distribution of myelin-associated glycoprotein and myelin basic protein in multiple sclerosis lesions. Ann Neurol. 1980 Feb;7(2):167–177. doi: 10.1002/ana.410070212. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Phase Behavior of Chloroplast and Microsomal Membranes during Leaf Senescence. Plant Physiol. 1978 Apr;61(4):639–643. doi: 10.1104/pp.61.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G. M. Multiple sclerosis. Annu Rev Neurosci. 1982;5:219–239. doi: 10.1146/annurev.ne.05.030182.001251. [DOI] [PubMed] [Google Scholar]
- Muranushi N., Takagi N., Muranishi S., Sezaki H. Effect of fatty acids and monoglycerides on permeability of lipid bilayer. Chem Phys Lipids. 1981 May;28(3):269–279. doi: 10.1016/0009-3084(81)90013-x. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- SALEM L. The role of long-range forces in the cohesion of lipoproteins. Can J Biochem Physiol. 1962 Sep;40:1287–1298. [PubMed] [Google Scholar]
- Shechter E., Letellier L., Gulik-Krzywicki G. Relations between structure and function in cytoplasmic membrane vesicles isolated from an Escherichia coli fatty-acid auxotroph. High-angle x-ray diffraction, freeze-etch electron microscopy and transport studies. Eur J Biochem. 1974 Nov 1;49(1):61–76. doi: 10.1111/j.1432-1033.1974.tb03811.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kamoshita S., Eto Y., Tourtellotte W. W., Gonatas J. O. Myelin in multiple sclerosis. Composition of myelin from normal-appearing white matter. Arch Neurol. 1973 May;28(5):293–297. doi: 10.1001/archneur.1973.00490230029002. [DOI] [PubMed] [Google Scholar]
- den Hartog Jager W. A. A histochemical study in Huntington's disease and control cases. Histochemistry. 1978 Dec 13;58(4):273–280. doi: 10.1007/BF00495383. [DOI] [PubMed] [Google Scholar]


