Abstract
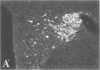
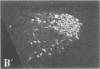
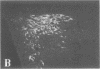
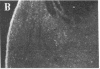
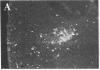
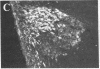
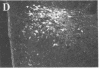
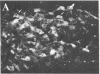
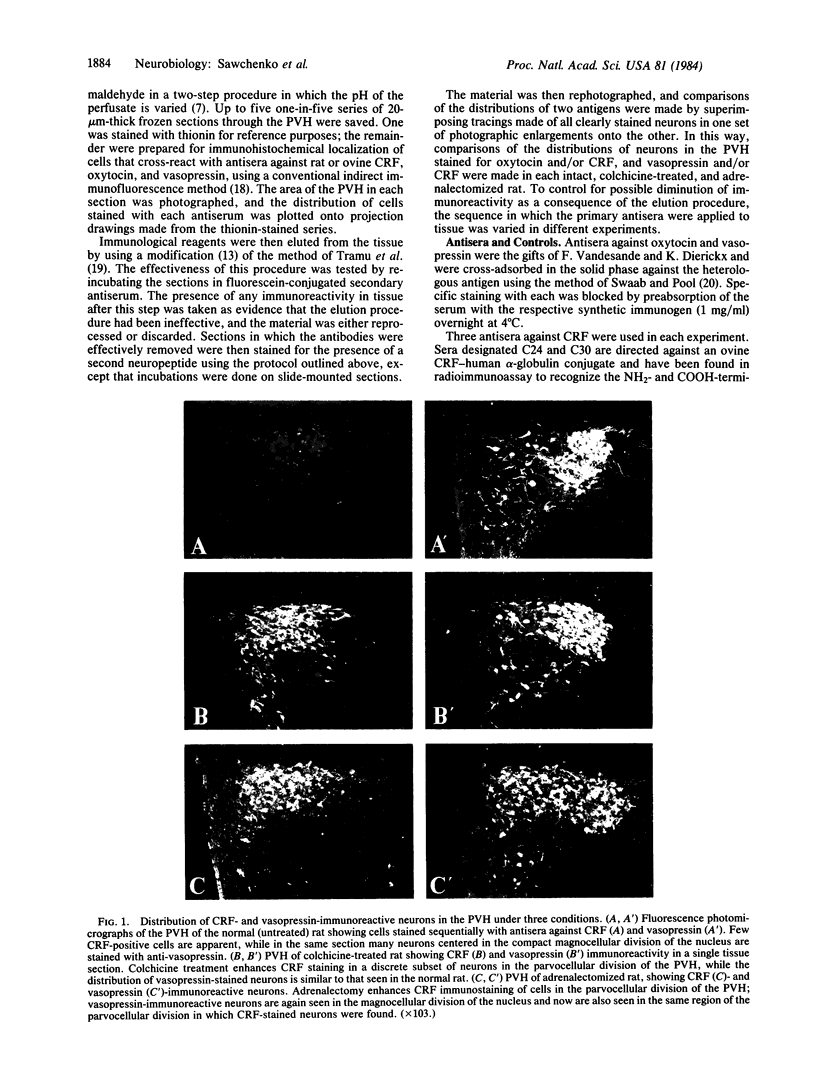
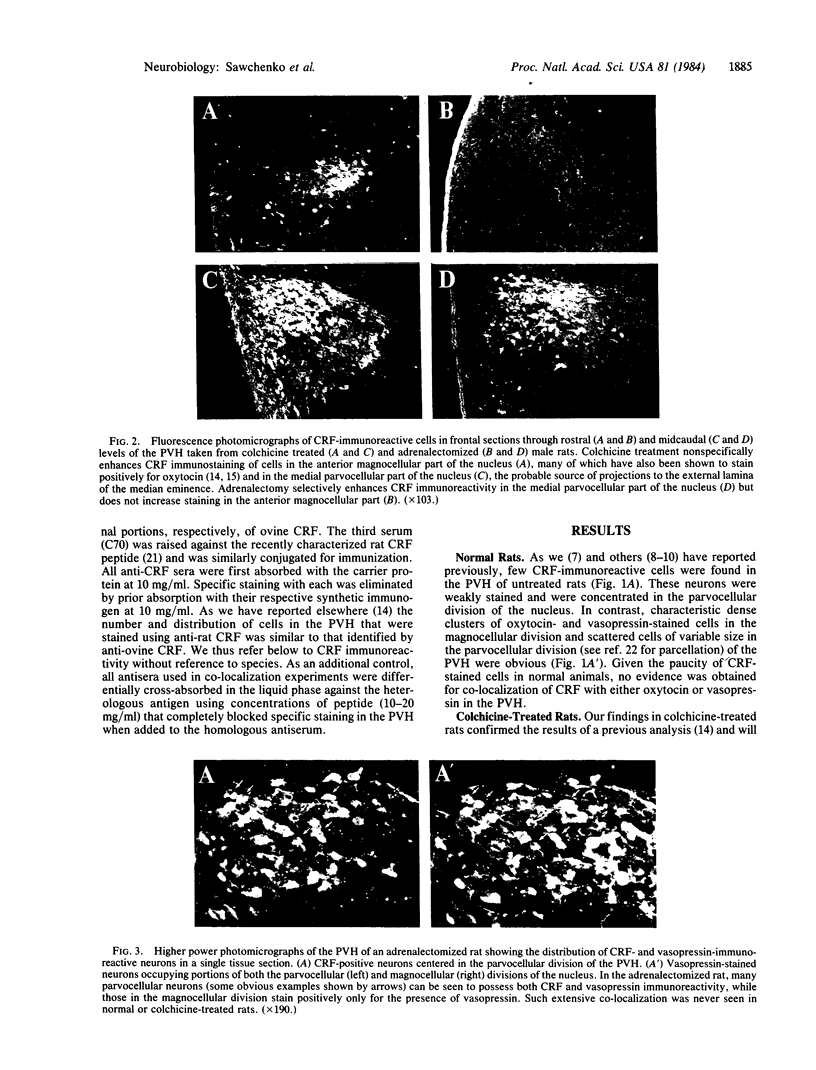
To clarify the anatomical organization that allows for the synergy of vasopressin and oxytocin with corticotropin-releasing factor (CRF) in promoting adrenocorticotropic hormone secretion from the anterior pituitary, immunohistochemical double staining methods were used to compare the distribution of these peptides in the hypothalamic paraventricular nucleus of normal, colchicine-treated, and adrenalectomized male rats. In untreated animals, a few CRF-stained cells were found in the parvocellular division of the paraventricular nucleus, while brightly stained oxytocin- and vasopressin-immunoreactive cells were centered in the magnocellular division. In animals treated with colchicine, and inhibitor of axonal transport, large numbers of CRF-stained cells were found in the parvocellular division of the nucleus, and 1-2% of these also stained with antivasopressin. As reported previously, a substantial number of oxytocin-stained cells, centered in a discrete anterior part of the magnocellular division, also expressed CRF immunoreactivity. In contrast, after adrenalectomy, CRF immunostaining of cells in the parvocellular division was enhanced selectively and greater than 70% of these cells also stained positively for vasopressin. The distribution of oxytocin-stained cells was not influenced by adrenalectomy. The unusual localization of vasopressin immunoreactivity in parvocellular neurosecretory neurons in the adrenalectomized rat suggests that a single population of cells can produce CRF and vasopressin, both of which are potent promoters of adrenocorticotropic hormone secretion. These findings indicate that there is a state-dependent plasticity in the expression of biologically active peptides by individual neuroendocrine neurons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. E., Battenberg E. L., Rivier J., Vale W. Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul Pept. 1982 Jun;4(1):43–48. doi: 10.1016/0167-0115(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Bugnon C., Fellmann D., Gouget A., Cardot J. Corticoliberin in rat brain: immunocytochemical identification and localization of a novel neuroglandular system. Neurosci Lett. 1982 May 17;30(1):25–30. doi: 10.1016/0304-3940(82)90006-4. [DOI] [PubMed] [Google Scholar]
- Burlet A., Tonon M. C., Tankosic P., Coy D., Vaudry H. Comparative immunocytochemical localization of corticotropin releasing factor (CRF-41) and neurohypophysial peptides in the brain of Brattleboro and Long-Evans rats. Neuroendocrinology. 1983 Jul;37(1):64–72. doi: 10.1159/000123517. [DOI] [PubMed] [Google Scholar]
- Deschepper C., Lotstra F., Vandesande F., Vanderhaeghen J. J. Cholecystokinin varies in the posterior pituitary and external median eminence of the rat according to factors affecting vasopressin and oxytocin. Life Sci. 1983 May 30;32(22):2571–2577. doi: 10.1016/0024-3205(83)90240-0. [DOI] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Fahrenkrug J., Tatemoto K., Mutt V., Werner S., Hulting A. L., Terenius L., Chang K. J. The PHI (PHI-27)/corticotropin-releasing factor/enkephalin immunoreactive hypothalamic neuron: possible morphological basis for integrated control of prolactin, corticotropin, and growth hormone secretion. Proc Natl Acad Sci U S A. 1983 Feb;80(3):895–898. doi: 10.1073/pnas.80.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechan R. M., Nestler J. L., Jacobson S. The tuberoinfundibular system of the rat as demonstrated by immunohistochemical localization of retrogradely transported wheat germ agglutinin (WGA) from the median eminence. Brain Res. 1982 Aug 5;245(1):1–15. doi: 10.1016/0006-8993(82)90334-1. [DOI] [PubMed] [Google Scholar]
- Luine V. N., Khylchevskaya R. I., McEwen B. S. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res. 1975 Mar 21;86(2):293–306. doi: 10.1016/0006-8993(75)90704-0. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Vigh S., Petrusz P., Schally A. V. The paraventriculo-infundibular corticotropin releasing factor (CRF) pathway as revealed by immunocytochemistry in long-term hypophysectomized or adrenalectomized rats. Regul Pept. 1983 Mar;5(4):295–305. doi: 10.1016/0167-0115(83)90287-2. [DOI] [PubMed] [Google Scholar]
- Moldow R. L., Fischman A. J. Hypothalamic CRF-like immunoreactivity in the rat after hypophysectomy or adrenalectomy. Peptides. 1982 Mar-Apr;3(2):143–147. doi: 10.1016/0196-9781(82)90043-2. [DOI] [PubMed] [Google Scholar]
- Rhodes C. H., Morrell J. I., Pfaff D. W. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981 May 1;198(1):45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- Rivier J., Spiess J., Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4851–4855. doi: 10.1073/pnas.80.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. A method for tracing biochemically defined pathways in the central nervous system using combined fluorescence retrograde transport and immunohistochemical techniques. Brain Res. 1981 Apr 6;210(1-2):31–51. doi: 10.1016/0006-8993(81)90882-9. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982 Mar 1;205(3):260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Stillman M. A., Recht L. D., Rosario S. L., Seif S. M., Robinson A. G., Zimmerman E. A. The effects of adrenalectomy and glucocorticoid replacement on vasopressin and vasopressin-neurophysin in the zona externa of the median eminence of the rat. Endocrinology. 1977 Jul;101(1):42–49. doi: 10.1210/endo-101-1-42. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Pool C. W. Specificity of oxytocin and vasopressin immunofluorescence. J Endocrinol. 1975 Aug;66(2):263–272. doi: 10.1677/joe.0.0660263. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Kuypers H. G. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980 Dec 1;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tramu G., Pillez A., Leonardelli J. An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem. 1978 Apr;26(4):322–324. doi: 10.1177/26.4.207771. [DOI] [PubMed] [Google Scholar]
- Turkelson C. M., Thomas C. R., Arimura A., Chang D., Chang J. K., Shimizu M. In vitro potentiation of the activity of synthetic ovine corticotropin-releasing factor by arginine vasopressin. Peptides. 1982 Mar-Apr;3(2):111–113. doi: 10.1016/0196-9781(82)90037-7. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brown M. R., Spiess J., Koob G., Swanson L., Bilezikjian L., Bloom F., Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;39:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vale W., Vaughan J., Smith M., Yamamoto G., Rivier J., Rivier C. Effects of synthetic ovine corticotropin-releasing factor, glucocorticoids, catecholamines, neurohypophysial peptides, and other substances on cultured corticotropic cells. Endocrinology. 1983 Sep;113(3):1121–1131. doi: 10.1210/endo-113-3-1121. [DOI] [PubMed] [Google Scholar]
- Vandesande F., Dierickx K., De Mey J. The origin of the vasopressinergic and oxytocinergic fibres of the external region of the median eminence of the rat hypophysis. Cell Tissue Res. 1977 Jun 13;180(4):443–452. doi: 10.1007/BF00220167. [DOI] [PubMed] [Google Scholar]
- Wiegand S. J., Price J. L. Cells of origin of the afferent fibers to the median eminence in the rat. J Comp Neurol. 1980 Jul 1;192(1):1–19. doi: 10.1002/cne.901920102. [DOI] [PubMed] [Google Scholar]