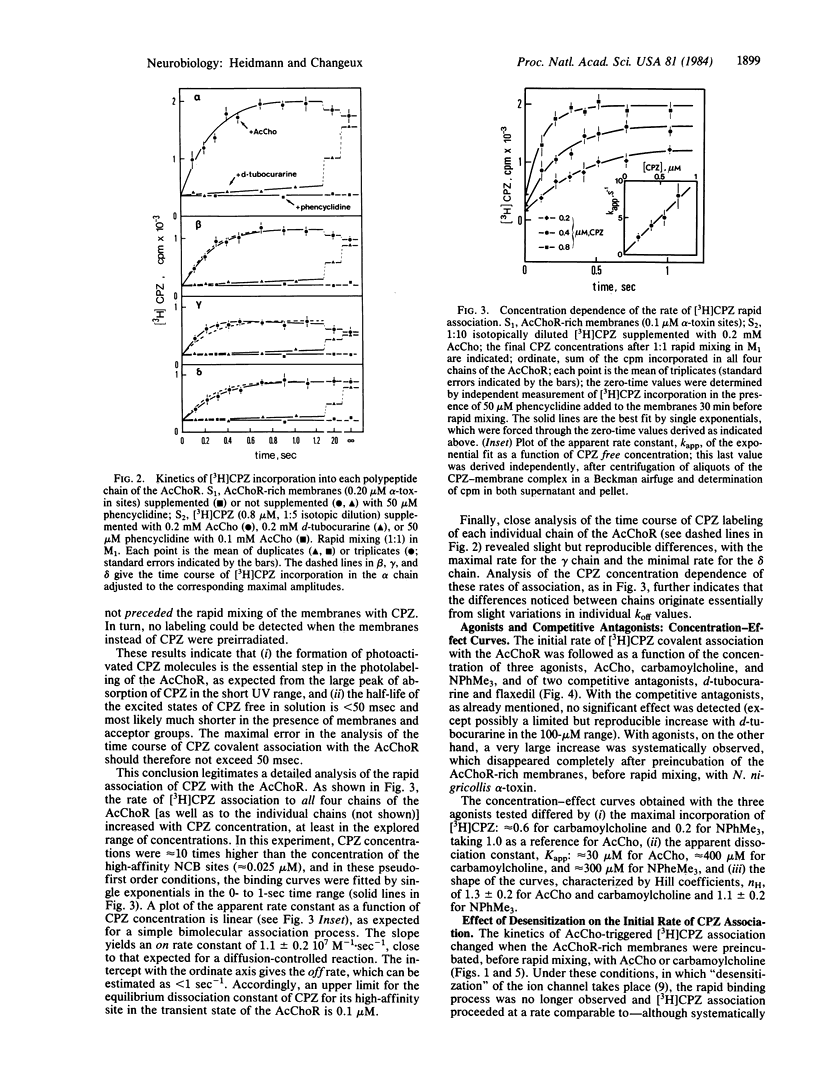
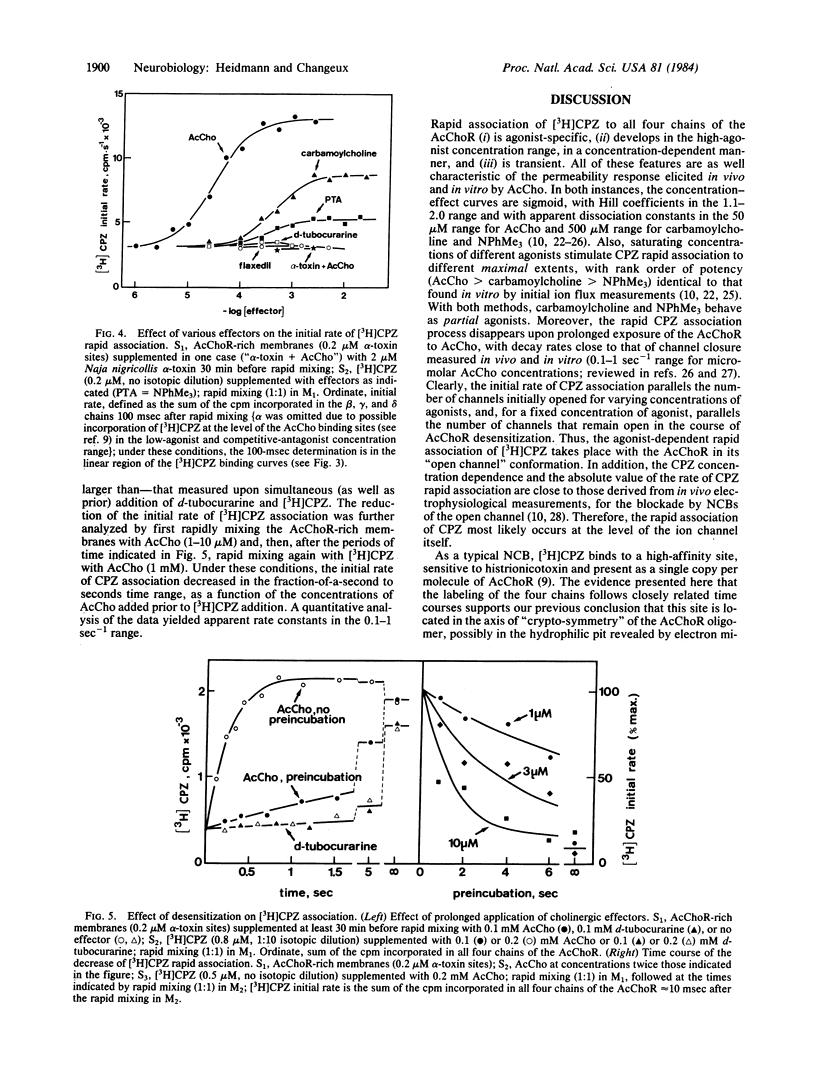
Abstract
A rapid-mixing photolabeling apparatus is developed to resolve the kinetics of association of the noncompetitive channel blocker [3H]chlorpromazine (CPZ) with the membrane-bound acetylcholine (AcCho) receptor from Torpedo marmorata and to photolabel its subunits in the 100-milli-seconds to seconds time range. Rapid mixing of AcCho and [3H]CPZ with the receptor followed by brief (less than 20 msec) UV irradiation results in the selective labeling of the four chains of the AcCho receptor, according to a rapid bimolecular association process close to diffusion-controlled. Rapid association is not observed with the competitive antagonists d-tubocurarine or flaxedil or the snake venom alpha-toxins. Its initial rate increases with agonist concentration, with maxima of 0.6 for carbamoylcholine and 0.2 for phenyltrimethylammonium taking 1 for AcCho, with apparent dissociation constants of 30 microM, 400 microM, and 300 microM for AcCho, carbamoylcholine, and phenyltrimethylammonium, respectively, and with sigmoid shape (Hill coefficients of 1.1-1.3). Under conditions in which the receptor "desensitizes" and the ionic channel closes (preincubation with AcCho), rapid [3H]CPZ association decreases in parallel. It is concluded that the agonist-dependent rapid association of [3H]CPZ takes place at the level of a site common to all five subunits, which lies within the ion channel and becomes accessible when the channel opens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Aronstam R. S., Eldefrawi A. T., Pessah I. N., Daly J. W., Albuquerque E. X., Eldefrawi M. E. Regulation of [3H]perhydrohistrionicotoxin binding to Torpedo ocellata electroplax by effectors of the acetylcholine receptor. J Biol Chem. 1981 Mar 25;256(6):2843–2850. [PubMed] [Google Scholar]
- Cartaud J., Benedetti E. L. A morphological study of the cholinergic receptor protein from Torpedo marmorata in its membrane environment and in its detergent-extracted purified form. J Cell Sci. 1978 Feb;29:313–337. doi: 10.1242/jcs.29.1.313. [DOI] [PubMed] [Google Scholar]
- Cash D. J., Hess G. P. Molecular mechanism of acetylcholine receptor-controlled ion translocation across cell membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):842–846. doi: 10.1073/pnas.77.2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. The acetylcholine receptor: an "allosteric" membrane protein. Harvey Lect. 1979 1980;75:85–254. [PubMed] [Google Scholar]
- Dunn S. M., Raftery M. A. Activation and desensitization of Torpedo acetylcholine receptor: evidence for separate binding sites. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6757–6761. doi: 10.1073/pnas.79.22.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünhagen H. H., Iwatsubo M., Changeux J. P. Fast kinetic studies on the interaction of cholinergic agonists with the membrane-bound acetylcholine receptor from Torpedo marmorata as revealed by quinacrine fluorescence. Eur J Biochem. 1977 Oct 17;80(1):225–242. doi: 10.1111/j.1432-1033.1977.tb11875.x. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Bernhardt J., Neumann E., Changeux J. P. Rapid kinetics of agonist binding and permeability response analyzed in parallel on acetylcholine receptor rich membranes from Torpedo marmorata. Biochemistry. 1983 Nov 8;22(23):5452–5459. doi: 10.1021/bi00292a029. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Oswald R. E., Changeux J. P. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Kaldany R. R., Karlin A. Reaction of quinacrine mustard with the acetylcholine receptor from Torpedo californica. J Biol Chem. 1983 May 25;258(10):6232–6242. [PubMed] [Google Scholar]
- Lindstrom J., Merlie J., Yogeeswaran G. Biochemical properties of acteylcholine receptor subunits from Torpedo californica. Biochemistry. 1979 Oct 16;18(21):4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- Muhn P., Hucho F. Covalent labeling of the acetylcholine receptor from Torpedo electric tissue with the channel blocker [3H]triphenylmethylphosphonium by ultraviolet irradiation. Biochemistry. 1983 Jan 18;22(2):421–425. doi: 10.1021/bi00271a028. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Boyd N. D., Cohen J. B. Conformations of Torpedo acetylcholine receptor associated with ion transport and desensitization. Biochemistry. 1982 Jul 6;21(14):3460–3467. doi: 10.1021/bi00257a032. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Cohen J. B. Permeability control by cholinergic receptors in Torpedo postsynaptic membranes: agonist dose-response relations measured at second and millisecond times. Biochemistry. 1980 Jun 10;19(12):2770–2779. doi: 10.1021/bi00553a036. [DOI] [PubMed] [Google Scholar]
- Oswald R. E., Changeux J. P. Selective labeling of the delta subunit of the acetylcholine receptor by a covalent local anesthetic. Biochemistry. 1981 Dec 8;20(25):7166–7174. doi: 10.1021/bi00528a018. [DOI] [PubMed] [Google Scholar]
- Oswald R. E. Effects of calcium on the binding of phencyclidine to acetylcholine receptor-rich membrane fragments from Torpedo californica electroplaque. J Neurochem. 1983 Oct;41(4):1077–1084. doi: 10.1111/j.1471-4159.1983.tb09054.x. [DOI] [PubMed] [Google Scholar]
- Oswald R. E., Heidmann T., Changeux J. P. Multiple affinity states for noncompetitive blockers revealed by [3H]phencyclidine binding to acetylcholine receptor rich membrane fragments from Torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3128–3136. doi: 10.1021/bi00282a015. [DOI] [PubMed] [Google Scholar]
- Oswald R., Changeux J. P. Ultraviolet light-induced labeling by noncompetitive blockers of the acetylcholine receptor from Torpedo marmorata. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3925–3929. doi: 10.1073/pnas.78.6.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Wennogle L. P., Changeux J. P. Factors regulating the susceptibility of the acetylcholine receptor protein to heat inactivation. FEBS Lett. 1979 Dec 15;108(2):489–494. doi: 10.1016/0014-5793(79)80595-5. [DOI] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Trautmann A. Curare can open and block ionic channels associated with cholinergic receptors. Nature. 1982 Jul 15;298(5871):272–275. doi: 10.1038/298272a0. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Changeux J. P. High affinity binding of alpha-bungarotoxin to the purified alpha-subunit and to its 27,000-dalton proteolytic peptide from Torpedo marmorata acetylcholine receptor. Requirement for sodium dodecyl sulfate. EMBO J. 1983;2(3):381–387. doi: 10.1002/j.1460-2075.1983.tb01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Takeyasu K., McNamee M. G. Activation and inactivation kinetics of Torpedo californica acetylcholine receptor in reconstituted membranes. Biochemistry. 1982 Oct 26;21(22):5384–5389. doi: 10.1021/bi00265a001. [DOI] [PubMed] [Google Scholar]
- Ziskind L., Dennis M. J. Depolarising effect of curare on embryonic rat muscles. Nature. 1978 Dec 7;276(5688):622–623. doi: 10.1038/276622a0. [DOI] [PubMed] [Google Scholar]