Abstract
Background
Assessment of left ventricular (LV) function with echocardiography is mandatory in patients with suspected heart failure (HF).
Objectives
To investigate if GPs were able to evaluate the LV function in patients at risk of developing or with established HF by using pocket-sized ultrasound (pUS).
Methods
Feasibility study in general practice, seven GPs in three different Norwegian primary care centres participated. Ninety-two patients with reduced or at risk of developing reduced LV function were examined by their own GP using pUS. The scan (<5 minute) was done as part of a routine appointment. A cardiologist examined the patients <30 minutes afterwards with both a laptop scanner and pUS. Measurements of the septal mitral annular excursion (sMAE) were compared.
Results
In 87% of the patients, the GPs were able to obtain a standard view and measure the sMAE. There was a non-significant mean difference in sMAE between GP pUS and cardiologist laptop scanner of −0.15 mm 95% confidence interval (−0.60 to 0.30) mm. A comparison of the pUS recordings and measurements of sMAE made by GP versus cardiologist revealed a non-significant mean difference with acceptable 95% limits of agreement (−0.26 ± 3.02 mm).
Conclusions
With tailored training, GPs were able to assess LV function with sMAE and pUS. pUS, as a supplement to the physical examination, may become an important tool in general practice.
Keywords: Echocardiography, general practice, heart failure, left ventricular dysfunction, ultrasound
Introduction
Heart failure (HF) is a common disease in the community setting.1 At the same time, the sensitivity of clinical signs is low and GPs have a limited toolbox available to provide a rapid diagnosis.2 Assessment of left ventricular (LV) function with echocardiography is mandatory in patients with suspected HF.3 However, most echocardiographic scanners are located in hospitals and operated by a limited number of highly trained personnel. In the study group on HF Awareness and Perception in Europe survey, only 50% of primary care physicians could obtain echocardiograms directly (16%) or via specialists (34%) within 1 month.4
HF is prevalent and according to the European Society of Cardiology, there are 15 million patients with HF among their member countries that comprise a population >900 million.3 HF accounts for 2% of national expenditure on health due to hospital admissions.5 To prevent emergency admissions and readmissions is thus a goal for the health authorities.
The development of pocket-sized, easy-to-use and affordable ultrasound machines might help spread the use of echocardiography to general practice. The accuracy of these scanners has been proven in the hands of experts.6 However, the ability of GPs to use these machines is not known. The recently published statement from the European Association of Echocardiography on the use of pocket-sized imaging devices emphasizes that handheld ultrasound represents a tool for fast initial cardiac assessment as a complement to the physical examination and that it may work in triaging of the patient in need of a complete echocardiographic examination.7
The standard way to assess LV systolic function is to measure the ejection fraction (LV-EF) with the Simpson's technique.8 However, this method requires good image quality for adequate tracing of the endocardial borders. The mitral annular excursion (MAE) is an easily obtainable surrogate measure of LV systolic function.9 Several studies have shown a high correlation between the MAE and LV-EF determined by biplane area–length method.10–12 An excursion >10 mm represents a normal LV-EF defined as EF 50%–55%.10,13 A reduction of MAE precedes that in circumferential systolic function in hypertensive cardiomyopathy and correlates with severity of valve disease in aortic stenosis.9 The MAE has been shown to be an independent prognostic variable for survival and has a negative correlation with brain natriuretic peptide (BNP).14,15
The aim of this study was to investigate if GPs, after an 8 hour training course, were able to do a focussed assessment of the LV function by measuring the septal mitral annular excursion (sMAE) in patients at risk of developing or with established HF, using pocket-sized ultrasound (pUS).
Methods
The study was performed in three different primary care centres in Norway. Altogether, seven GPs participated in the study.
Subjects
The study population (Table 1) comprised 92 patients, 61 (66%) males, median (range) age was 72.5 (38–88) years. Patients with at least one of the following characteristics—systolic HF (32%), earlier myocardial infarction (63%) or arterial hypertension (40%) were recruited to the study by their GPs. The GPs searched their archives for patients with a relevant International Classification of Primary Care diagnosis: K75 myocardial infarction, K77 HF and K87 hypertension complicated. Some of the patients had a previous echocardiographic examination, in such case, the GP was specifically told not to checkup these results. There were no specific exclusion criteria and no selection according to echogenicity. The three different centres included 19, 21 and 52 patients. The different GPs included between 3 and 52 patients. Written informed consent was obtained from all participants.
Table 1.
Basic characteristics of 92 study participants
| N (%)a | |
| Age, years median (range) | 72.5 (38–88) |
| Male | 61 (66%) |
| HF | 29 (32%) |
| Previous myocardial infarction | 58 (63%) |
| Hypertension | 37 (40%) |
Data are N (%) unless specified.
Ultrasound imaging examination
None of the participating doctors had any experience with performing echocardiography, although all of them had observed demonstrations of echocardiographic examinations. All GPs covered the entire field of general practice.
The participating GPs received a total of 8 hours of supervised training. The lecturer throughout this course was a cardiologist certified in echocardiography in accordance with the requirements specified by the Norwegian Medical Association. In the first 4 hours of the course, the GPs were taught the very basic principles of theoretical echocardiography and anatomy of the heart as visualized by ultrasound. The GPs were taught how to examine the patients positioned in the left lateral decubitus position and how to make an optimal apical four-chamber view. They were also taught how to analyse the LV function by measuring the sMAE. The next 4 hours consisted of practical training, including practicing on each other and on 3–5 patients. After the supervised training period, they had the possibility to practice with the scanner on their own for a week before the study started.
All patients were examined in the GPs' primary care centres. Images were obtained by the seven GPs using a pUS scanner capable of B-mode and colour flow imaging (Vscan; GE Healthcare, Horten, Norway). An algorithm enables automatic storage and looping of a cardiac cycle without electorcardiogram signal. An examination contained a recording of an apical four-chamber view. This added no more than 5 minutes to the consultation.
In an adjacent examination room, a full reference examination was made by a cardiologist certified in echocardiography using a laptop scanner (Vivid I; GE Healthcare). This examination was performed immediately (<30 minutes) after the GP finished his/her examination and according to a complete protocol for a transthoracic echocardiogram including two-dimensional imaging, Doppler and tissue Doppler recordings. Immediately after having performed the complete scan, the cardiologist also examined all the patients with the pUS scanner.
Data analyses
The pUS recordings were exported to the commercially available software Vscan Gateway (GE Healthcare) on a standard laptop PC. The GP did the analysis of the sMAE in the loop of one cardiac cycle. The measurement was made by scrolling the two-dimensional loop from end diastole to end systole and then measuring the total displacement of the septal part of the mitral annulus, which represents the total displacement throughout a cardiac cycle (Fig. 1). The Vscan has a limited field of view of up to 75° for black and white imaging. Due to dropouts and out-of-plane movement of the lateral part of the mitral ring, the septal part was chosen for all measurements. The recordings were analysed by the GP that actually examined the patient. All the recordings acquired by the GPs were also analysed offline by a second cardiologist certified and experienced in echocardiography.
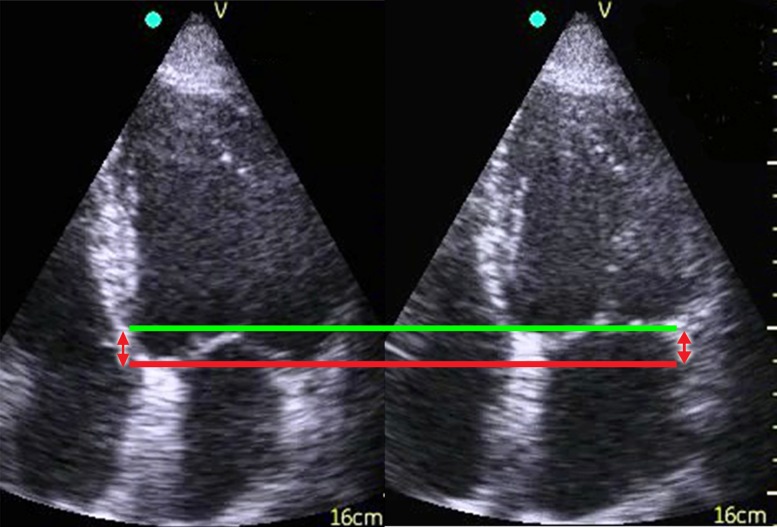
Figure 1.
Two-dimensional recording of the left ventricle in end diastole (left) and end systole (right). The red line indicates the position of the mitral annular septum in end diastole and the green line, the position in end systole. The distance between the lines (red arrows) represents the total distance/excursion by the septal part of the mitral annulus through a complete heart cycle
The reference examinations performed on the laptop scanner were exported to the commercially available Echo PAC PC version BT 09 (GE Healthcare, Horten, Norway). In this full reference examination, the M-mode recording of the mitral annulus was measured according to the method performed in previous studies (Fig. 2).11,12 Only septal measurements were used in the comparisons with the pUS recordings.
Figure 2.
M-mode registration of sMAE. The distance between the red lines (arrow) indicates the total distance/excursion by the septal part of the mitral annulus through a complete heart cycle
Statistics
The null hypothesis corresponded to no difference between the sMAE measured by the GPs and a cardiologist. Including 92 patients resulted in power >90% at the 5% significance level for detecting a difference in 2 mm in sMAE, assuming one SD of 1.51 mm.
Continuous values are expressed as median and range. Because the differences were normal distributed, we used paired t-test to compare sMAE measured by the GPs and the cardiologist. When comparing the two different scanners, the laptop scanner is considered the gold standard and the results are reported as mean difference and 95% confidence interval (CI). Bland and Altman analysis was used to assess agreement between different operators and measurements of sMAE with pUS.16 The coefficient of repeatability and mean error of the sMAE were calculated for pocket-sized data. The inter-observer coefficient of repeatability was defined as 1.96 SD of the differences in repeated measurements. The mean error was defined as the absolute difference in between two sets of observations divided by the mean of the observations ± SD%. Statistical analyses were performed using SPSS PASW Statistics 18.0.
Results
Table 1 shows the basic characteristics of the patients. In 80 (87%) of patients, the GPs were able to obtain a four-chamber view of such a quality that they were able to measure the sMAE. Seven of the remaining recordings were of such a bad quality that further analysis was impossible and in five of the recordings, an image was recorded instead of a loop. The pUS examination was performed without delaying the standard consultation. A full reference examination and a pocket-sized recording were obtained in all 92 patients by the cardiologist. The LV-EF ranged from 25% to 70% with a median of 52%. The sMAE ranged from 4.0 to 15.0 mm with a median of 10.0 mm.
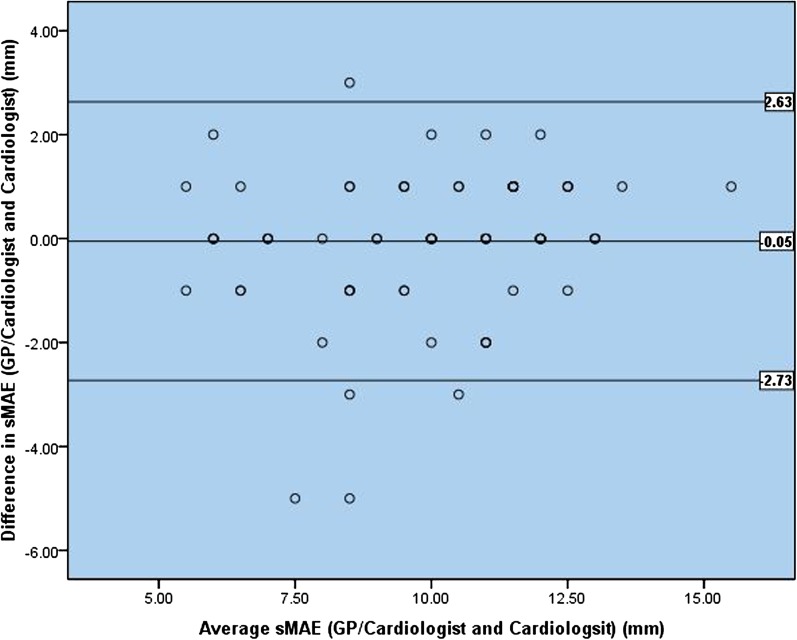
There was a non-significant mean difference in sMAE between GP pUS and cardiologist laptop scanner of −0.15 mm 95% CI (−0.60 to 0.30) mm. Comparing the two different scanners, when both were operated by the first cardiologist, there was a non-significant mean difference in sMAE of 0.11 mm 95% CI (−0.10 to 0.32) mm. Comparing the measurement of the sMAE made by the GPs and the sMAE made by the cardiologist both using pUS, the 95% limit of agreement was −0.26 ± 3.02 mm and there was virtually no bias or trend in the data (Fig. 3). When a second cardiologist did the offline analysis on the recordings acquired by the GPs, the 95% limit of agreement narrowed to −0.05 ± 2.68 mm (Fig. 4). There were no significant differences in measurements of sMAE, neither between the different operators or the different scanners. The measurements for each of the three centres are presented in Table 2. The study was not designed or powered to perform statistical analysis for each centre and data are presented as mean difference ± SD.
Figure 3.
Ninety-five per cent limits of agreement between measurements of sMAE with pUS (Vscan) performed and analysed by the GPs versus the cardiologist
Figure 4.
Ninety-five per cent limits of agreement between measurements of sMAE with pUS (Vscan) performed by the GPs and analysed by the second cardiologist versus the cardiologist
Table 2.
The mean difference for the different comparisons for the total study population and for each of the three centres
| Total (n = 92) | Centre 1 (n = 21) | Centre 2 (n = 52) | Centre 3 (n = 19) | |
| pUS GP versus laptopa | −0.15 mm (−0.60 to 0.30) | 0.77 mm (±1.56) | −0.73 mm (±1.96) | 1.18 mm (±2.09) |
| pUS GP/cardiologist versus laptopa | 0.08 mm (−0.29 to 0.44) | 0.65 mm (±1.32) | −0.24 mm (±1.68) | 0.64 mm (±1.43) |
| pUS cardiologist versus laptopa | 0.11 mm (−0.10 to 0.32) | 0.57 mm (±1.29) | −0.09 mm (±0.93) | 0.19 mm (±0.66) |
| pUS GP versus pUS cardiologistb | −0.26 mm (±3.02) | 0.12 mm (±1.41) | −0.65 mm (±1.37) | 1.0 mm (±1.79) |
| pUS GP/cardiologist versus pUS cardiologistb | −0.05 mm (±2.68) | 0.00 mm (±1.50) | −0.18 mm (±1.38) | 0.45 mm (±1.04) |
In comparisons between the different scanners, the laptop scanner is considered the gold standard and the 95% CI of the mean difference is given for the total population and mean difference ± SD for each of the centres. In comparisons between different operators both using pUS, the 95% limit of agreement is given for the total population and mean difference ± SD for each of the centres. pUS GP, pUS performed and analysed by the GP; pUS cardiologist, pUS performed and analysed by the cardiologist; pUS GP/cardiologist, pUS performed by the GP and analysed by the second cardiologist; laptop, echocardiography performed and analysed by the cardiologist using a laptop scanner.
Data are mean difference and 95% CI for the total population and mean difference ± SD for each centre.
Data are mean difference and 95% limits of agreement for the total population and mean difference ± SD for each centre.
Sensitivity and specificity of GP operated pUS to detect a reduced LV function defined as an sMAE <10 mm measured by the cardiologist with a laptop scanner was 83.3% 95% CI (66.4–92.7) and 77.6% 95% CI (64.1–87.0) respectively. The negative and positive predictive value were 88.4% and 69.4%, respectively. When the offline analysis of GP recordings was performed by the second cardiologist, sensitivity and specificity increased to 77.4% 95% CI (60.2–88.6) and 85.4% 95% CI (72.8–92.8) and the negative and positive predictive value 85.4% and 77.4%, respectively.
The inter-observer coefficient of repeatability for pocket-sized recordings and measurement of sMAE was 3.1 mm and the mean error was 12.2% ± 11.5%.
Discussion
In this study, we have shown that it is possible for GPs, after a limited period of focussed training, to use a pUS scanner to assess a surrogate marker for global LV function in 87% of the patients with or at risk of developing reduced LV function. The pUS examination could easily be performed in 5 minutes during a routine consultation in the GPs office. The sMAE was selected as a robust parameter and easily obtainable measurement of the LV function. There was no significant difference between measurements of sMAE obtained by the GPs and a cardiologist, neither with a laptop scanner and the gold standard M-mode nor with pUS with its limited frame rate. When operated by the cardiologist, pUS offered the same accuracy as a laptop scanner when evaluating the sMAE.
GPs have limited tools available for rapid assessment of patients with dyspnoea and there is a lack of access to echocardiography.1,2,17 To our knowledge, our study is the first that has assessed whether a number of GPs, in different primary care centres, can do an examination with pUS to assess LV function in patients at risk of developing or with established HF. The training of GPs in our study was limited but specifically tailored to the information they should obtain from this new class of devices. This strategy is fully supported by the recent guidelines issued from the European Association of Echocardiography regarding the use of these new machines in that users should focus their examination to answer a specific question and use this as a tool to support their physical examination.7 The scan was integrated in the physical examination and was performed in <5 minutes. The sensitivity and negative predictive values were high.
In agreement with our study, previous studies have shown that echocardiography may provide information even in the hands of inexperienced users. Decara et al. 18 showed how hand-carried ultrasound devices used by medical students significantly resulted in a more accurate bedside diagnosis. Lucas et al. 19 showed that the diagnostic accuracy of hand-carried ultrasound devices performed by hospitalists after a brief training programme was moderate to excellent for six important cardiac abnormalities. Lipczynska et al. 20 found that hand-carried ultrasound examinations of the heart performed by an internist with 4 weeks of training can provide important prognostic information, independent of BNP. Similar to the duration of training in our study, Vignon et al. 21 found that intensive care resident doctors were able to rule out LV dysfunction (LV-EF < 50%) by eyeballing after an 8 hours focussed training programme.
According to the current guidelines, the recommended method for evaluating LV function is the EF calculated from the biplane method of discs (modified Simpsons' rule).8 This is not an easy method for an inexperienced users mainly because endocardial definition requires a high image quality. Tracing of endocardial borders in both end diastole and end systole in two imaging planes are required to calculate the LV-EF in this method [EF = 100 × (end-diastolic volume − end-systolic volume)/end-diastolic volume]. Analysing the MAE is another established method for evaluating LV function.10–12 This is a simple method, it is highly reproducible and it is feasible even in patients with poor image quality.10,22 No studies have been found to report inter-observer variability for measurement of MAE using pocket-sized scanners, only high-end scanners operated by experts. In one study, Thorstensen et al. 22 averaged M-mode excursion measurements in four points (septal, lateral, inferior and anterior wall) and reported 4% mean error and a coefficient of repeatability of 1.6 mm. Yuda et al. 10 reported 6.4% mean error, also using M-mode measurements. Using a speckle tracking approach, Tsang et al. 23 reported mean errors of 12.2% in apical four-chamber images and 12.7% in two-chamber images. In our study, calculating the mean error and coefficient of repeatability between the GP and the first cardiologist yields 12.1% and 3.08 mm.
The GPs had a limited amount of training and did only septal measurements of the MAE from four-chamber two-dimensional images as opposed to others that used the average of four measure points with M-mode along the mitral ring from the four and two chamber view.11,12 However, Pai et al. 24 reported an excellent correlation (r = 0.93) with septal measurements of the MAE from four-chamber two-dimensional images compared to radionuclide EFs (Multi Gated Acquisition Scan).
One limitation in our study is that the number of GPs was only seven, a higher number of participating GPs would have increased the generalizability. A further limitation is that in 13% of the patients, the GP was not able to measure the sMAE, this was mainly related to technical problems and would probably be a minor problem with further training. The first and second cardiologist was blinded to the GPs measurement of sMAE with pUS, but the first cardiologist was not blinded to his own M-mode measurements with the laptop scanner. This could have influenced the agreement between the two different scanners.
A normal sMAE does not rule out the possibility of the patient having cardiac dyspnoea and is not a substitute for a full echocardiographic examination by a cardiologist. The MAE is a surrogate marker of LV function, predominantly systolic function but also diastolic, and should be evaluated in the context of other variables such as valvular function. Yet, an examination with pUS is available at the GP office any time off-day and may allow an earlier and more correct care, both when systolic dysfunction is shown and when this is ruled out.
Further work should focus on the added value of pUS as a supportive tool in decision making in primary care and to determine whether the use of ultrasound in dyspnoeic patients improves diagnostics of HF and reduces the number of emergency admissions. Future work should also introduce software that may aid inexperienced users in getting better images and make automatic measurements of the MAE.25 It is also important to formalize training programmes in order to avoid misuse.
Conclusions
With tailored training, GPs in this study were able to assess LV function with sMAE and pUS. pUS, as a supplement to the physical examination, may become an important tool in general practice, but further studies are needed to see if this improves diagnostics of HF in general practice.
Declaration
Funding: Norwegian University of Science and Technology and Research Council of Norway.
Ethical approval: Regional Committee for Medical and Health Research Ethics (reference number: 4.2009.257) and the Norwegian Social Science Data Service (reference number: 21549/2/LT).
Conflict of interest: none.
Acknowledgments
We thank the physicians and nurses at all the three primary care centres for assistance with inclusions and data collection.
References
- 1.Carmona M, Garcia-Olmos LM, Alberquilla A, et al. Heart failure in the family practice: a study of the prevalence and co-morbidity. Fam Pract. 2011;28:128–33. doi: 10.1093/fampra/cmq084. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–8. [PubMed] [Google Scholar]
- 3.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 4.Remme WJ, McMurray JJ, Hobbs FD, et al. Awareness and perception of heart failure among European cardiologists, internists, geriatricians, and primary care physicians. Eur Heart J. 2008;29:1739–52. doi: 10.1093/eurheartj/ehn196. [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, Jenkins A, Buchan S, et al. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail. 2002;4:361–71. doi: 10.1016/s1388-9842(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 6.Prinz C, Voigt JU. Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr. 2011;24:111–6. doi: 10.1016/j.echo.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Sicari R, Galderisi M, Voigt JU, et al. The use of pocket-size imaging devices: a position statement of the European Association of Echocardiography. Eur J Echocardiogr. 2011;12:85–7. doi: 10.1093/ejechocard/jeq184. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Zaca V, Ballo P, Galderisi M, Mondillo S. Echocardiography in the assessment of left ventricular longitudinal systolic function: current methodology and clinical applications. Heart Fail Rev. 2010;15:23–37. doi: 10.1007/s10741-009-9147-9. [DOI] [PubMed] [Google Scholar]
- 10.Yuda S, Inaba Y, Fujii S, et al. Assessment of left ventricular ejection fraction using long-axis systolic function is independent of image quality: a study of tissue Doppler imaging and m-mode echocardiography. Echocardiography. 2006;23:846–52. doi: 10.1111/j.1540-8175.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- 11.Alam M, Hoglund C, Thorstrand C. Longitudinal systolic shortening of the left ventricle: an echocardiographic study in subjects with and without preserved global function. Clin Physiol. 1992;12:443–52. doi: 10.1111/j.1475-097x.1992.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 12.Alam M, Hoglund C, Thorstrand C, Philip A. Atrioventricular plane displacement in severe congestive heart failure following dilated cardiomyopathy or myocardial infarction. J Intern Med. 1990;228:569–75. doi: 10.1111/j.1365-2796.1990.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 13.Alam M, Hoglund C, Thorstrand C, Hellekant C. Haemodynamic significance of the atrioventricular plane displacement in patients with coronary artery disease. Eur Heart J. 1992;13:194–200. doi: 10.1093/oxfordjournals.eurheartj.a060146. [DOI] [PubMed] [Google Scholar]
- 14.Svealv BG, Olofsson EL, Andersson B. Ventricular long-axis function is of major importance for long-term survival in patients with heart failure. Heart. 2008;94:284–9. doi: 10.1136/hrt.2006.106294. [DOI] [PubMed] [Google Scholar]
- 15.Elnoamany MF, Abdelhameed AK. Mitral annular motion as a surrogate for left ventricular function: correlation with brain natriuretic peptide levels. Eur J Echocardiogr. 2006;7:187–98. doi: 10.1016/j.euje.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 17.Sim V, Hampton D, Phillips C, et al. The use of brain natriuretic peptide as a screening test for left ventricular systolic dysfunction—cost-effectiveness in relation to open access echocardiography. Fam Pract. 2003;20:570–4. doi: 10.1093/fampra/cmg513. [DOI] [PubMed] [Google Scholar]
- 18.Decara JM, Kirkpatrick JN, Spencer KT, et al. Use of hand-carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses. J Am Soc Echocardiogr. 2005;18:257–63. doi: 10.1016/j.echo.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of hospitalist-performed hand-carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4:340–9. doi: 10.1002/jhm.438. [DOI] [PubMed] [Google Scholar]
- 20.Lipczynska M, Szymanski P, Klisiewicz A, Hoffman P. Hand-carried echocardiography in heart failure and heart failure risk population: a community based prospective study. J Am Soc Echocardiogr. 2011;24:125–31. doi: 10.1016/j.echo.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Vignon P, Dugard A, Abraham J, et al. Focused training for goal-oriented hand-held echocardiography performed by noncardiologist residents in the intensive care unit. Intensive Care Med. 2007;33:1795–9. doi: 10.1007/s00134-007-0742-8. [DOI] [PubMed] [Google Scholar]
- 22.Thorstensen A, Dalen H, Amundsen BH, Aase SA, Stoylen A. Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr. 2010;11:149–56. doi: 10.1093/ejechocard/jep188. [DOI] [PubMed] [Google Scholar]
- 23.Tsang W, Ahmad H, Patel AR, et al. Rapid estimation of left ventricular function using echocardiographic speckle-tracking of mitral annular displacement. J Am Soc Echocardiogr. 2010;23:511–5. doi: 10.1016/j.echo.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Pai RG, Bodenheimer MM, Pai SM, Koss JH, Adamick RD. Usefulness of systolic excursion of the mitral anulus as an index of left ventricular systolic function. Am J Cardiol. 1991;67:222–4. doi: 10.1016/0002-9149(91)90453-r. [DOI] [PubMed] [Google Scholar]
- 25.Snare SR, Mjolstad OC, Orderud F, Haugen BO, Torp H. Fast automatic measurement of mitral annulus excursion using a pocket-sized ultrasound system. Ultrasound Med Biol. 2011;37:617–31. doi: 10.1016/j.ultrasmedbio.2010.12.012. [DOI] [PubMed] [Google Scholar]