Abstract
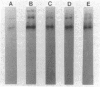
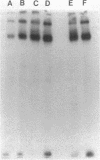
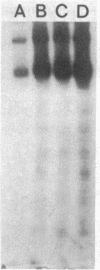
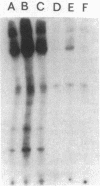
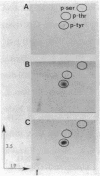
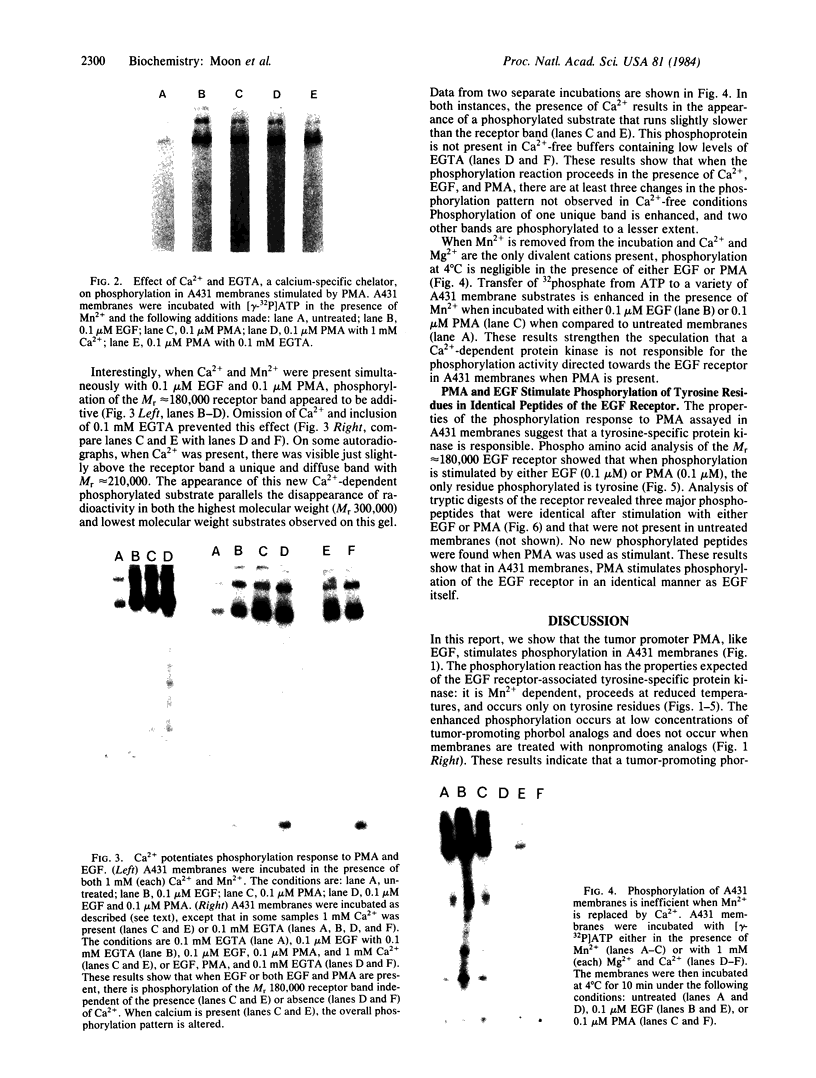
Incubation of membranes prepared from A431 cells with either epidermal growth factor (EGF) or phorbol 12-myristate 13-acetate (PMA) stimulates the transfer of 32phosphate from [gamma-32P]ATP into 8-10 membrane proteins. The major phosphorylated protein migrates on NaDodSO4/polyacrylamide gels with an apparent Mr of 180,000, corresponding to the previously identified EGF receptor. Stimulation of EGF receptor phosphorylation by PMA does not require Ca2+, suggesting that prior activation of protein kinase C is not a prerequisite for phosphate transfer. PMA-enhanced phosphorylation proceeds at 4 degrees C and requires Mn2+, both properties of tyrosine-specific protein kinases. Phospho amino acid analysis of the Mr 180,000 receptor band shows that only tyrosine residues are phosphorylated when A431 membranes are treated with either EGF or PMA. Moreover, proteolysis reveals that these residues are located in the same peptides of the receptor. These results demonstrate that a potent tumor-promoting phorbol ester can mimic a critical early response usually elicited by EGF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Ashendel C. L., Staller J. M., Boutwell R. K. Identification of a calcium- and phospholipid- dependent phorbol ester binding activity in the soluble fraction of mouse tissues. Biochem Biophys Res Commun. 1983 Feb 28;111(1):340–345. doi: 10.1016/s0006-291x(83)80157-0. [DOI] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Carlin C. R., Knowles B. B. Identity of human epidermal growth factor (EGF) receptor with glycoprotein SA-7: evidence for differential phosphorylation of the two components of the EGF receptor from A431 cells. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5026–5030. doi: 10.1073/pnas.79.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Phillips P. D., Knowles B. B., Cristofalo V. J. Diminished in vitro tyrosine kinase activity of the EGF receptor of senescent human fibroblasts. Nature. 1983 Dec 8;306(5943):617–620. doi: 10.1038/306617a0. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978 Nov 23;276(5686):409–410. doi: 10.1038/276409a0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Glaser L. Proteolytic cleavage of epidermal growth factor receptor. A Ca2+-dependent, sulfhydryl-sensitive proteolytic system in A431 cells. J Biol Chem. 1982 Aug 25;257(16):9845–9848. [PubMed] [Google Scholar]
- Cassel D., Glaser L. Proteolytic cleavage of epidermal growth factor receptor. A Ca2+-dependent, sulfhydryl-sensitive proteolytic system in A431 cells. J Biol Chem. 1982 Aug 25;257(16):9845–9848. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol. 1984 Jan;4(1):30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Lazar C. S. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981 Sep 24;293(5830):305–307. doi: 10.1038/293305a0. [DOI] [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Ek B., Rönnstrand L. Characterization of the receptor for platelet-derived growth factor on human fibroblasts. Demonstration of an intimate relationship with a 185,000-Dalton substrate for the platelet-derived growth factor-stimulated kinase. J Biol Chem. 1983 Aug 25;258(16):10054–10061. [PubMed] [Google Scholar]
- Horowitz A. D., Greenebaum E., Weinstein I. B. Identification of receptors for phorbol ester tumor promoters in intact mammalian cells and of an inhibitor of receptor binding in biologic fluids. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2315–2319. doi: 10.1073/pnas.78.4.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Resolution of high and low affinity epidermal growth factor receptors. Inhibition of high affinity component by low temperature, cycloheximide, and phorbol esters. J Biol Chem. 1982 Mar 25;257(6):3053–3060. [PubMed] [Google Scholar]
- King L. E., Jr, Carpenter G., Cohen S. Characterization by electrophoresis of epidermal growth factor stimulated phosphorylation using A-431 membranes. Biochemistry. 1980 Apr 1;19(7):1524–1528. doi: 10.1021/bi00548a040. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Laszlo A., Radke K., Chin S., Bissell M. J. Tumor promoters alter gene expression and protein phosphorylation in avian cells in culture. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6241–6245. doi: 10.1073/pnas.78.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Fox C. F. Controlled proteolysis of EGF receptors: evidence for transmembrane distribution of the EGF binding and phosphate acceptor sites. J Supramol Struct. 1980;14(4):461–471. doi: 10.1002/jss.400140405. [DOI] [PubMed] [Google Scholar]
- Magun B. E., Matrisian L. M., Bowden G. T. Epidermal growth factor. Ability of tumor promoter to alter its degradation, receptor affinity and receptor number. J Biol Chem. 1980 Jul 10;255(13):6373–6381. [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K. Tyrosine-specific protein kinase activity associated with p105 of avian sarcoma virus PRCII. Virology. 1981 Feb;109(1):223–228. doi: 10.1016/0042-6822(81)90493-1. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palfrey H. C., Rothlein J. E., Greengard P. Calmodulin-dependent protein kinase and associated substrates in Torpedo electric organ. J Biol Chem. 1983 Aug 10;258(15):9496–9503. [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D. S. Inhibition of epidermal growth factor binding to mouse embryonal carcinoma cells by phorbol esters mediated by specific phorbol ester receptors. J Biol Chem. 1981 Aug 10;256(15):7958–7966. [PubMed] [Google Scholar]
- Sando J. J., Young M. C. Identification of high-affinity phorbol ester receptor in cytosol of EL4 thymoma cells: requirement for calcium, magnesium, and phospholipids. Proc Natl Acad Sci U S A. 1983 May;80(9):2642–2646. doi: 10.1073/pnas.80.9.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schreiber A. B., Yarden Y., Schlessinger J. A non-mitogenic analogue of epidermal growth factor enhances the phosphorylation of endogenous membrane proteins. Biochem Biophys Res Commun. 1981 Jul 30;101(2):517–523. doi: 10.1016/0006-291x(81)91290-0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Todaro G. J. Specific high affinity cell membrane receptors for biologically active phorbol and ingenol esters. Nature. 1980 Dec 4;288(5790):451–455. doi: 10.1038/288451a0. [DOI] [PubMed] [Google Scholar]
- Solanki V., Slaga T. J. Specific binding of phorbol ester tumor promoters to intact primary epidermal cells from Sencar mice. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2549–2553. doi: 10.1073/pnas.78.4.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth D. K., Niedel J. E., Pastan I. Vinculin, a cytoskeletal substrate of protein kinase C. J Biol Chem. 1983 Oct 10;258(19):11423–11426. [PubMed] [Google Scholar]
- Yeaton R. W., Lipari M. T., Fox C. F. Calcium-mediated degradation of epidermal growth factor receptor in dislodged A431 cells and membrane preparations. J Biol Chem. 1983 Aug 10;258(15):9254–9261. [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Patterson E., Michael D., Elgjo K., Hennings H. Stimulated DNA synthesis in mouse epidermal cell cultures treated with 12-O-tetradecanoyl-phorbol-13-acetate. Cancer Res. 1976 Nov;36(11 Pt 1):4062–4068. [PubMed] [Google Scholar]