Abstract
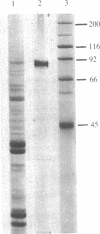
A DNA polymerase has been purified >3,000-fold from the chloroplasts of pea plants by chromatography on DEAE-cellulose, phosphocellulose, single-stranded DNA-agarose, and sedimentation in a glycerol gradient. Electrophoretic analysis on polyacrylamide gels in the presence of sodium dodecyl sulfate indicates that the final fraction contained a single discernible protein band of 90,000 daltons. Gel filtration on Sephacryl S-200 and glycerol gradient sedimentation under nondenaturing conditions demonstrate that the chloroplast DNA polymerase has a native molecular mass of approximately 87,000 daltons. The purified polymerase lacks any associated nuclease activity. The enzyme activity is inhibited by N-ethylmaleimide (74% at 1.0 mM) and ethidium bromide (90% at 0.23 mM) and is resistant to aphidicolin. The purified enzyme is totally dependent on the presence of added DNA, has an absolute requirement for Mg2+ (12 mM optimal), is stimulated by K+ (120 mM optimal), and requires all four deoxynucleoside triphosphates for maximum activity. Native DNA which has been degraded to a limited extent with DNase I is the most efficient template.
Keywords: circular DNA, DNA replication
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Bottomley W., Smith H. J., Bogorad L. RNA polymerases of maize: partial purification and properties of the chloroplast enzyme. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2412–2416. doi: 10.1073/pnas.68.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Korn D. DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells. J Biol Chem. 1977 Sep 25;252(18):6528–6535. [PubMed] [Google Scholar]
- Kolodner R. D., Tewari K. K. Chloroplast DNA from higher plants replicates by both the Cairns and the rolling circle mechanism. Nature. 1975 Aug 28;256(5520):708–711. doi: 10.1038/256708a0. [DOI] [PubMed] [Google Scholar]
- Kolodner R. D., Tewari K. K. Denaturation mapping studies on the circular chloroplast deoxyribonucleic acid from pea leaves. J Biol Chem. 1975 Jul 10;250(13):4888–4895. [PubMed] [Google Scholar]
- LEHMAN I. R., RICHARDSON C. C. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. IV. AN EXONUCLEASE ACTIVITY PRESENT IN PURIFIED PREPARATIONS OF DEOXYRIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1964 Jan;239:233–241. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyer R. R., Simpson M. V. Deoxyribonucleic acid biosynthesis in mitochondria. Purification and general properties of rat liver mitochondrial deoxyribonucleic acid polymerase. J Biol Chem. 1970 Jul 10;245(13):3426–3435. [PubMed] [Google Scholar]
- Misumi M., Weissbach A. The isolation and characterization of DNA polymerase alpha from spinach. J Biol Chem. 1982 Mar 10;257(5):2323–2329. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Schrecker A. W., Brinkman B. J., Gallo R. C. DNA polymerase gamma of human lymphoblasts. Biochemistry. 1977 Jun 28;16(13):2866–2873. doi: 10.1021/bi00632a010. [DOI] [PubMed] [Google Scholar]
- Sala F., Amileni A. R., Parisi B., Spadari S. A gamma-like DNA polymerase in spinach chloroplasts. Eur J Biochem. 1980 Nov;112(2):211–217. doi: 10.1111/j.1432-1033.1980.tb07196.x. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. Purification and properties of nuclear and cytoplasmic deoxyribonucleic acid polymerases from human KB cells. J Biol Chem. 1972 Aug 25;247(16):5026–5033. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Tewari K. K., Wildman S. G. DNA polymerase in isolated tobacco chloroplasts and nature of the polymerized product. Proc Natl Acad Sci U S A. 1967 Aug;58(2):689–696. doi: 10.1073/pnas.58.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Weissbach A. Deoxyribonucleic acid synthesis in isolated chloroplasts and chloroplast extracts of maize. Biochemistry. 1982 Jul 6;21(14):3334–3343. doi: 10.1021/bi00257a014. [DOI] [PubMed] [Google Scholar]