Abstract
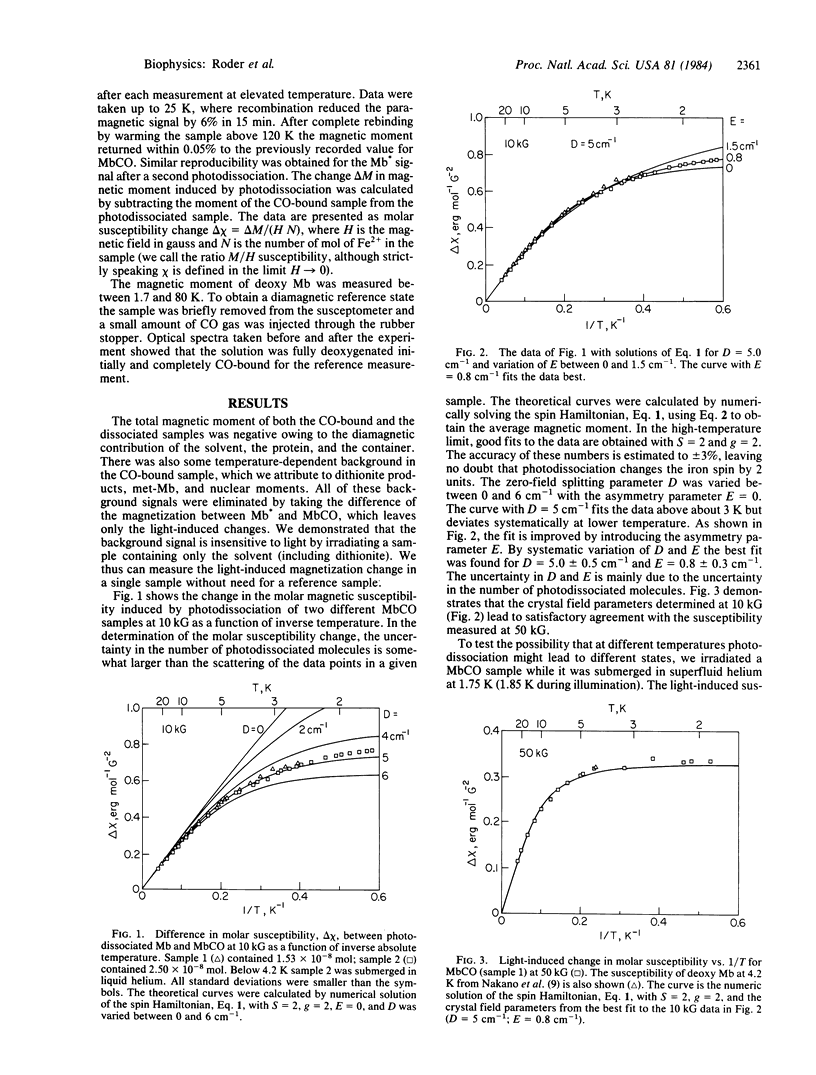
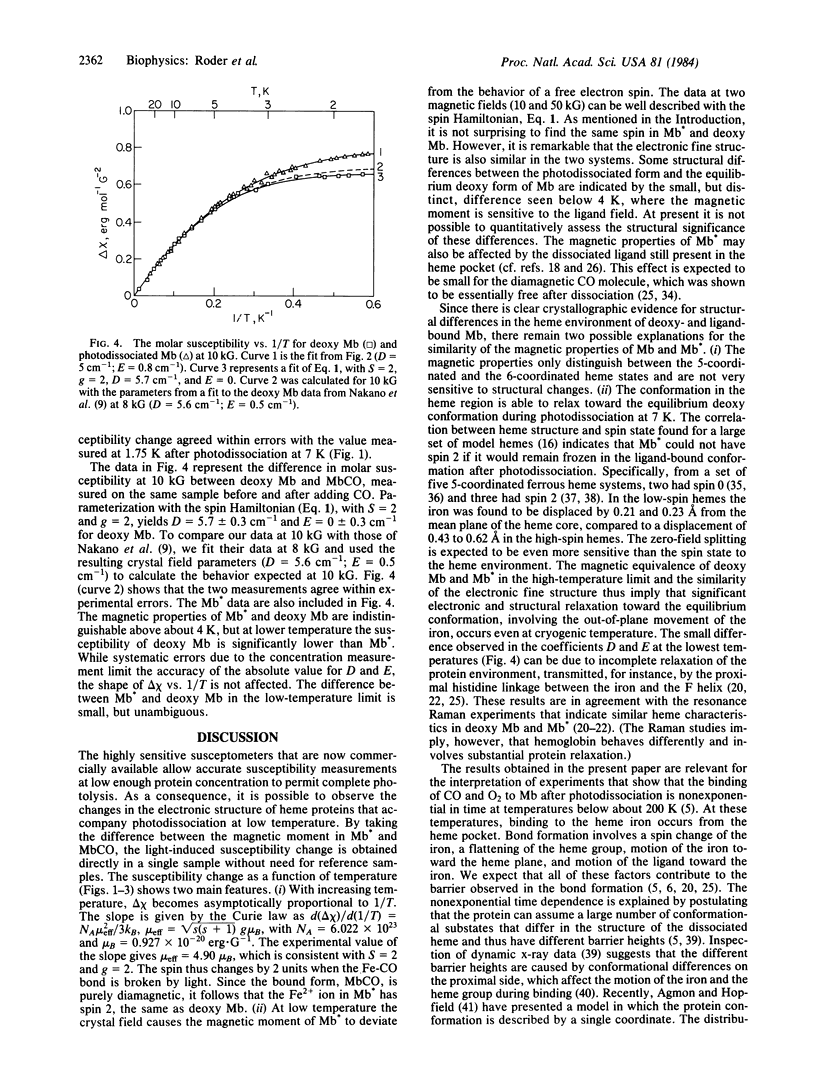
The magnetic susceptibility of photodissociated carbon monoxy myoglobin has been measured over the temperature range from 1.7 to 25 K at 10 and 50 kG with a superconducting susceptometer. The spin and the crystal field parameters of the iron ion were extracted by a spin Hamiltonian approach. Under equivalent conditions the magnetic susceptibility of deoxy myoglobin was measured. In both experiments the CO-bound protein was used as a diamagnetic reference. Above about 5 K the metastable photolysed state and the equilibrium deoxy form of myoglobin are magnetically indistinguishable and can be fitted with S = 2 and g = 2. The transition from spin 0 to spin 2 and the conformational changes known to accompany the electronic change thus also occur after photolysis at low temperature. At temperatures below 5 K, differences become apparent, indicating a somewhat smaller zero-field splitting in the photoproduct as compared to the ligand-free state at equilibrium. In qualitative agreement with observations made by other techniques, the data imply that even at 1.7 K substantial structural relaxation occurs in the heme region of myoglobin after photodissociation. The results are important for the interpretation of the ligand binding kinetics after flash photolysis at low temperature and contribute to the understanding of the relationship between electronic structure and function in heme proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alben J. O., Beece D., Bowne S. F., Doster W., Eisenstein L., Frauenfelder H., Good D., McDonald J. D., Marden M. C., Moh P. P. Infrared spectroscopy of photodissociated carboxymyoglobin at low temperatures. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3744–3748. doi: 10.1073/pnas.79.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Bade D., Parak F. Investigation of the electronic term scheme of deoxygenated human haemoglobin by a least squares fit procedure using simultaneously magnetic susceptibility and Mössbauer data. Biophys Struct Mech. 1976 Dec 22;2(3):219–231. doi: 10.1007/BF00535368. [DOI] [PubMed] [Google Scholar]
- Chance B., Fischetti R., Powers L. Structure and kinetics of the photoproduct of carboxymyoglobin at low temperatures: an X-ray absorption study. Biochemistry. 1983 Aug 2;22(16):3820–3829. doi: 10.1021/bi00285a017. [DOI] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Rousseau D. L., Ondrias M. R., Stepnoski R. A. Transient Raman study of hemoglobin: structural dependence of the iron-histidine linkage. Science. 1982 Dec 17;218(4578):1244–1246. doi: 10.1126/science.7146910. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Stepnoski R. A., Stavola M., Ondrias M. R., Cone R. L. Ligation and quaternary structure induced changes in the heme pocket of hemoglobin: a transient resonance Raman study. Biochemistry. 1982 Apr 27;21(9):2022–2028. doi: 10.1021/bi00538a008. [DOI] [PubMed] [Google Scholar]
- Iizuka T., Yamamoto H., Kotani M., Yonetani T. Low temperature photodissociation of hemoproteins: carbon monoxide complex of myoglobin and hemoglobin. Biochim Biophys Acta. 1974 Nov 5;371(1):126–139. doi: 10.1016/0005-2795(74)90161-5. [DOI] [PubMed] [Google Scholar]
- Marcolin H. E., Reschke R., Trautwein A. Mössbauer spectroscopic investigations of photodissociated myoglobin-CO at low temperatures. Eur J Biochem. 1979 May 2;96(1):119–123. doi: 10.1111/j.1432-1033.1979.tb13020.x. [DOI] [PubMed] [Google Scholar]
- Nakano N., Otsuka J., Tasaki A. Fine structure of iron ion in deoxymyoglobin and deoxyhemoglobin. Biochim Biophys Acta. 1971 Apr 27;236(1):222–233. doi: 10.1016/0005-2795(71)90169-3. [DOI] [PubMed] [Google Scholar]
- Nakano N., Otsuka J., Tasaki A. Paramagnetic anisotropy measurements on a single crystal of deoxyhemoglobin. Biochim Biophys Acta. 1972 Sep 29;278(2):355–371. doi: 10.1016/0005-2795(72)90240-1. [DOI] [PubMed] [Google Scholar]
- Norvell J. C., Nunes A. C., Schoenborn B. P. Neutron diffraction analysis of myoglobin: structure of the carbon monoxide derivative. Science. 1975 Nov 7;190(4214):568–570. doi: 10.1126/science.1188354. [DOI] [PubMed] [Google Scholar]
- Ondrias M. R., Friedman J. M., Rousseau D. L. Metastable species of hemoglobin: room temperature transients and cryogenically trapped intermediates. Science. 1983 May 6;220(4597):615–617. doi: 10.1126/science.6836305. [DOI] [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Shelnutt J. A., Simon S. R. Quaternary-transformation-induced changes at the heme in deoxyhemoglobins. Biochemistry. 1982 Jul 6;21(14):3428–3437. doi: 10.1021/bi00257a028. [DOI] [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Simon S. R. Resonance Raman spectra of photodissociated carbonmonoxy hemoglobin and deoxy hemoglobin at 10 K. J Biol Chem. 1983 May 10;258(9):5638–5642. [PubMed] [Google Scholar]
- Pauling L., Coryell C. D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936 Apr;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Phillips S. E. Structure of oxymyoglobin. Nature. 1978 May 18;273(5659):247–248. doi: 10.1038/273247a0. [DOI] [PubMed] [Google Scholar]
- Scheidt W. R., Frisse M. E. Nitrosylmetalloporphyrins. II. Synthesis and molecular stereochemistry of mitrosyl-alpha, beta, gamma, delta,-tetraphenylporphinatoiron (ii) J Am Chem Soc. 1975 Jan 8;97(1):17–21. doi: 10.1021/ja00834a005. [DOI] [PubMed] [Google Scholar]
- Spartalian K., Lang G., Yonetani T. Low temperature photodissociation studies of ferrous hemoglobin and myoglobin complexes by Mössbauer spectroscopy. Biochim Biophys Acta. 1976 Apr 23;428(2):281–290. doi: 10.1016/0304-4165(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]


