Abstract
Glutamate interacts with ionotropic and metabotropic glutamate receptors (mGluRs). Whereas the entorhinal cortex (EC) is a principal structure involved in learning and memory, the roles of mGluRs in synaptic transmission in the EC have not been completely determined. Here, we show that activation of group II mGluRs (mGluR II) induced robust depression of glutamatergic transmission in the EC. The mGluR II–induced depression was due to a selective reduction of presynaptic release probability without alterations of the quantal size and the number of release sites. The mechanisms underlying mGluR II–mediated suppression of glutamate release included the inhibition of presynaptic release machinery and the depression of presynaptic P/Q-type Ca2+ channels. Whereas mGluR II–induced depression required the function of Gαi/o proteins, protein kinase A (PKA) pathway was only involved in mGluR II–mediated inhibition of release machinery and thereby partially required for mGluR II–induced inhibition of glutamate release. Presynaptic stimulation at 5 Hz for 10 min also induced depression of glutamatergic transmission via activation of presynaptic mGluR II suggesting an endogenous role for mGluR II in modulating glutamatergic transmission.
Keywords: entorhinal cortex, glutamate receptor, G-protein, plasticity, synaptic transmission
Introduction
Glutamate is the principal excitatory neurotransmitter in the brain where it interacts with ionotropic (NMDA, AMPA, and kainate) and metabotropic glutamate receptors (mGluRs) (Dingledine et al. 1999; Riedel et al. 2003). Whereas the ionotropic glutamate receptors mediate the fast excitatory synaptic transmission, the mGluRs usually play a modulatory role in the brain. The mGluRs are G protein–coupled receptors that are linked to various intracellular second messenger cascades (Conn and Pin 1997; Pin and Acher 2002; Niswender and Conn 2010). Group I mGluRs (mGluR1 and mGluR5) are coupled to Gq/11. Activation of these receptors results in the activation of phospholipase C (PLC) leading to an increase in intracellular Ca2+ release and activation of protein kinase C (PKC) (Conn and Pin 1997; Pin and Acher 2002). Group II (mGluR2 and mGluR3) and group III (mGluR4 and mGluR6–8) mGluRs are coupled to Gαi/o proteins. These 2 groups of receptors are negatively linked to adenylate cyclase (AC) leading to a reduction in the intracellular level of cyclic AMP and an inhibition of protein kinase A (PKA) (Conn and Pin 1997; Pin and Acher 2002). The functions of mGluRs are involved in modulation of physiological functions such as synaptic plasticity, learning and memory, and neurological diseases including mental retardation, autism, Alzheimer's disease, Parkinson's disease, stroke, epilepsy, and drug addiction (Alexander and Godwin 2006; Luscher and Huber 2010; Niswender and Conn 2010). However, the cellular and molecular mechanisms of mGluRs in these physiological functions and neurological disorders are not completely elucidated.
The entorhinal cortex (EC) is part of a network in the limbic system that is closely involved in the consolidation and recall of memories (Haist et al. 2001; Squire et al. 2004; Dolcos et al. 2005; Steffenach et al. 2005), Alzheimer's disease (Hyman et al. 1984; Kotzbauer et al. 2001), schizophrenia (Falkai et al. 1988; Arnold et al. 1991; Joyal et al. 2002; Prasad et al. 2004), and temporal lobe epilepsy (Spencer and Spencer 1994; Avoli et al. 2002). Anatomically, the EC mediates the majority of the connections between the hippocampus and other cortical areas (Witter et al. 1989; Witter, Naber, et al. 2000). Afferents from the olfactory structures, parasubiculum, perirhinal cortex, claustrum, amygdala, and neurons in the deep layers of the EC (layers V–VI) converge onto the superficial layers (layer II/III) of the EC (Witter et al. 1989; Burwell 2000), whereas the axons of principal neurons in layer II form the major component of perforant path that innervates the dentate gyrus and CA3 (Steward and Scoville 1976) and those of the pyramidal neurons in layer III form the temporoammonic pathway that synapses onto the distal dendrites of pyramidal neurons in the CA1 and subiculum (Steward and Scoville 1976; Witter, Naber, et al. 2000; Witter, Wouterlood, et al. 2000). The output from the hippocampus is then projected to the deep layers of the EC that relay information back to the superficial layers (Kohler 1986; Dolorfo and Amaral 1998a, 1998b; van Haeften et al. 2003) and to other cortical areas (Witter et al. 1989). The EC expresses mGluRs including mGluR2 (Shigemoto et al. 1992; Ohishi et al. 1993b; Fotuhi et al. 1994), mGluR3 (Ohishi et al. 1993b; Fotuhi et al. 1994), mGluR4 (Phillips et al. 1997), and mGluR5 (Fotuhi et al. 1994). Functionally, activation of group I mGluRs in the EC facilitates persistent firing (Yoshida et al. 2008), inhibits glutamatergic transmission (Iserhot et al. 2004), and exerts diverse effects on γ-aminobutyric acid (GABA)ergic transmission (Deng et al. 2010). Furthermore, activation of group III mGluRs enhances glutamate release (Evans et al. 2000, 2001) and depresses spontaneous inhibition (Woodhall et al. 2001). However, the actions of group II mGluRs (denoted as mGluR II thereafter) in the EC have not been explicitly determined although they are implicated in modulation of synaptic transmission (Iserhot et al. 2004). In the present study, we examined the effects of mGluR II on glutamatergic transmission in the EC. We focused on layer III pyramidal neurons for the following 2 reasons that are relevant to the functions of mGluRs in the EC. First, mGluRs modulate consolidation of memory (Riedel et al. 2003), and the axons of the layer III pyramidal neurons form temporoammonic pathway, which is required for consolidation of memory (Remondes and Schuman 2004). Second, both the EC (Spencer and Spencer 1994; Avoli et al. 2002) and the mGluRs (Alexander and Godwin 2006; Niswender and Conn 2010) are involved in epilepsy and selective loss of layer III pyramidal neurons in the EC has been observed in epileptic animals (Du and Schwarcz 1992; Du et al. 1993) highlighting the importance of these neurons in epilepsy. Our results demonstrate that activation of mGluR II induces powerful depression of glutamatergic transmission in the EC via inhibition of presynaptic glutamate release probability. Interaction with the release machinery and inhibition of presynaptic P/Q-type Ca2+ channels contribute to mGluR II–induced depression, which requires the function of Gαi/o but partially depends on PKA pathway.
Materials and Methods
Slice Preparation
Horizontal brain slices (400 μm) including the EC, subiculum, and hippocampus were cut using a vibrating blade microtome (VT1000S; Leica, Wetzlar, Germany) from 15- to 20-day-old Sprague Dawley rats as described previously (Deng and Lei 2006, 2007, 2008; Deng et al. 2007). Briefly, after being deeply anesthetized with isoflurane, rats were decapitated and their brains were dissected out in ice-cold saline solution that contained (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 5.0 MgCl2, and 10 glucose, saturated with 95% O2 and 5% CO2 (pH 7.4). Slices were initially incubated in the above solution at 35 °C for 40 min for recovery and then kept at room temperature (∼24 °C) until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee.
Recordings of Synaptic Currents
Whole-cell patch-clamp recordings using an Axopatch 200B or 2 Multiclamp 700B amplifiers in voltage-clamp mode from in vitro entorhinal slices were used for experiments. Layer III pyramidal neurons in the medial EC were visually identified with infrared video microscopy and differential interference contrast optics (Deng et al. 2006, 2009; Lei et al. 2007; Xiao et al. 2009). Recording electrodes were filled with the solution containing (in mM) 100 Cs-gluconate, 0.6 ethyleneglycol-bis(2-aminoethylether)-N,N,N′,N′-tetra acetic acid, 5 MgCl2, 8 NaCl, 2 ATP2Na, 0.3 GTPNa, 40 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 1 QX-314 (pH 7.3). The extracellular solution comprised (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2, and 10 glucose (saturated with 95% O2 and 5% CO2, pH 7.4) unless stated otherwise. Bicuculline (10 μM) was included in the extracellular solution to block GABAA receptors. To prevent the propagation of epileptic activity in the presence of bicuculline, a cut was made between layer III and layer V with a microknife (catalog number: RS-6242, Roboz Surgical Instrument Company, Gaithersburg, MD) under a microscope before the slices were transferred to the recording chamber. The holding potential was at −65 mV unless stated otherwise. AMPA receptor–mediated excitatory postsynaptic currents (EPSCs) were evoked by placing a stimulation electrode in layer III about 200 μm from the recorded neuron. The duration of the stimulation was 3 ms and the stimulation intensity was adjusted to evoke synaptic currents reliably at individual synapses. For the isolation of NMDA EPSCs, the extracellular solution contained 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 μM) to block AMPA/kainate receptors and bicuculline (10 μM) to block GABAA receptors, and the holding potential was at +40 mV. Series resistance was rigorously monitored by the delivery of 5-mV voltage steps after each evoked current. Experiments were discontinued if the series resistance changed by >15%. Miniature AMPA EPSCs (mEPSCs) were recorded from layer III pyramidal cells of the EC in the presence of tetrodotoxin (TTX, 1 μM). Data were filtered at 2 kHz, digitized at 10 kHz, acquired online, and analyzed after-line using pCLAMP 9 software (Molecular Devices, Sunnyvale, CA). The recorded mEPSCs were analyzed afterward using Mini Analysis 6.0.1 (Synaptosoft Inc., Decatur, GA). To avoid potential desensitization, one slice was limited to only one application of mGluR II agonist and only one cell was recorded from each slice.
Recordings of Evoked Field Potentials
The recording methods for evoked field potentials were described previously (Deng and Lei 2007). Briefly, an extracellular recording pipette (2–5 MΩ) containing the extracellular solution was positioned in layer III of the EC and a concentric bipolar stimulation electrode (Frederick Haer and Company) was placed ∼200 μm from the recording electrode in layer III. Constant current pulses were delivered using a stimulus generator (WPI, Model A300) and a stimulus isolation unit (Model A360). The stimulation intensity was adjusted to obtain an amplitude of the field potentials within 50–80% of the maximal amplitude and was kept constant throughout the experiment. Evoked field potentials were filtered at 1 Hz and digitized at 10 kHz. The slopes of the evoked field potentials were measured using the program Clampfit (Axon Instruments).
Variance–Mean Analysis
Variance–mean (V–M) analysis was conducted by the methods described previously (Clements and Silver 2000; Clements 2003; Silver 2003). AMPA EPSC amplitudes were measured as the peak current minus the mean baseline current immediately (averaged over 5 ms) preceding each stimulation artifact. Mean amplitude (M) was averaged from 60 successive EPSCs recorded in different extracellular Ca2+ concentrations to alter release probability after stabilization of the EPSCs at each Ca2+ concentration. V was calculated as the square of the standard deviation of the 60 EPSCs amplitudes in each condition after correction for baseline variance. To ensure that postsynaptic AMPA receptors were responding to a nonsaturating concentration of glutamate, which is a requirement of V–M analysis, all experiments were conducted in a low concentration of DNQX (100 nM). The relationship between V and M can be described by the equation: V = qM − M2/N, where q is the quantal size and N is the number of release sites.
Data Analysis
Data are presented as the means ± standard error of the mean. Concentration–response curve of LY354740 was fit by Hill equation: I = Imax × {1/[1 + (EC50/[ligand])n]}, where Imax is the maximum response, EC50 is the concentration of ligand producing a half-maximal response, and n is the Hill coefficient. Coefficient of variation (CV) was calculated by the equation, CV = SD/X, where SD is the standard deviation and X is the mean of 20 consecutive AMPA EPSCs. The paired-pulse ratio (PPR) was calculated as the mean P2/mean P1, where P1 was the amplitude of first evoked current and P2 was the amplitude of the second synaptic current, measured after subtraction of the remaining P1 “tail” current (Kim and Alger 2001; Lei and McBain 2003). For mEPSC cumulative probability plots, events recorded for 4 min before LY354740 application and 4 min after the maximal effect of LY354740 were selected. Same bin size (100 ms for frequency and 1 pA for amplitude) was used to analyze data from control and LY354740 treatment. Kolmogorov–Smirnov test was used to assess the significance of the cumulative probability plots. Student's paired or unpaired t-test or analysis of variance was used for statistical analysis as appropriate; P values are reported throughout the text and significance was set as P < 0.05. N numbers in the text represent the number of cells examined unless stated otherwise.
Chemicals
LY354740, LY341495, (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl) glycine (DCG-IV), DNQX, dl-2-amino-5-phosphonopentanoic acid (dl-AVP), KT5720, and MDL-12,330A were purchased from Tocris Cookson Inc. (Ellisville, MO). ω-Conotoxin (ω-CgTx-GVIA) and ω-agatoxin IVA (ω-Aga-IVA) were products of Alomone Labs (Jerusalem, Israel). GDP-β-S and Rp-cAMPS were purchased from Enzo Life Sciences International, Inc. (Plymouth Meeting, PA). Other chemicals were products of Sigma-Aldrich (St Louis, MO).
Results
Activation of mGluR II Inhibits Glutamatergic Transmission but Does Not Modulate GABAergic Transmission in the EC
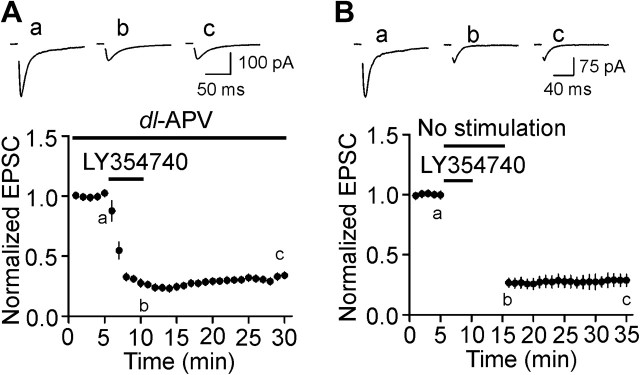
We initially tested the roles of mGluR II in glutamatergic transmission by recording evoked AMPA EPSCs from layer III pyramidal neurons in the EC. Bath application of LY354740 (3 μM), a specific agonist of mGluR II, for 5 min induced remarkable depression of the amplitudes of evoked AMPA EPSCs (19 ± 2% of control, n = 9, P < 0.001, Fig. 1A). The inhibition of AMPA EPSCs occurred within a couple of minutes after the beginning of the application of LY354740 and persisted for at least 20 min (29 ± 6% of control, n = 9, P < 0.001, Fig. 1A). To test whether LY354740-induced depression was mediated by mGluR II, we used LY341495, the specific antagonist for mGluR II. Bath application of LY341495 (3 μM) by itself did not significantly change AMPA EPSC amplitudes (n = 7, P = 0.23, Fig. 1B) but completely blocked LY354740-induced depression (n = 7, P = 0.98, Fig. 1B) demonstrating the requirement of mGluR II. The EC50 value of LY354740 was measured to be 0.025 μM (Fig. 1C). We used DCG-IV, another mGluR II agonist to further confirm the roles of mGluR II. Bath application of DCG-IV (3 μM) also inhibited AMPA EPSCs (38 ± 7% of control, n = 6, P < 0.001, Fig. 1D). In the presence of mGluR II antagonist LY341495 (3 μM), bath application of DCG-IV failed to induce depression (95 ± 5% of control, n = 5, P = 0.43, Fig. 1D). These data together demonstrate that activation of mGluR II depresses glutamatergic transmission in the EC.
Figure 1.
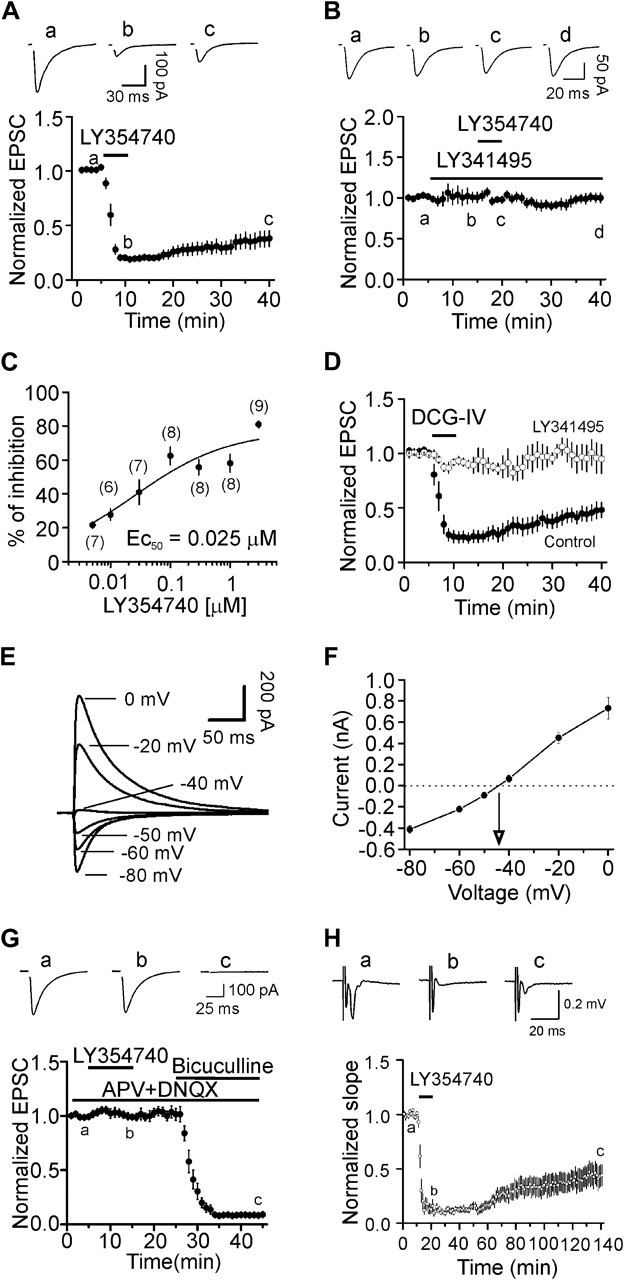
Activation of mGluR II inhibits glutamatergic transmission but exerts no effects on GABAergic transmission in layer III pyramidal neurons. (A) Bath application of LY354740 (3 μM) inhibited AMPA EPSCs. Upper panel shows the average of 10 EPSCs recorded at different time points in the figure (same for the rest of the figures). Stimulation artifacts were deleted for clarity. Lower panel shows the time course of the inhibition induced by LY354740. AMPA EPSC amplitudes at different time points were normalized to the average of the AMPA EPSCs recorded for 5 min before application of LY354740. (B) Bath application of the specific mGluR II antagonist, LY341495 (3 μM), blocked the inhibition induced by LY354740. Upper panel shows the averaged trace of 10 EPSCs recorded at different time points in the figure. (C) Concentration–response curve of LY354740. The numbers in the parentheses are the number of cells used for each concentration. (D) Bath application of DCG-IV (3 μM), another mGluR II agonist, inhibited AMPA EPSCs but failed to inhibit AMPA EPSCs in the presence of mGluR II antagonist, LY341495. (E) IPSCs recorded from a layer III pyramidal neuron at different holding potentials in the intracellular solution containing Cs+-gluconate and extracellular solution supplemented with DNQX (10 μM) and dl-APV (50 μM). (F), Voltage–current relationship averaged from 5 neurons. (G) Bath application of LY354740 (3 μM) had no effects on IPSCs recorded at –70 mV. At the end of the experiments, application of bicuculline (10 μM) completely blocked IPSCs indicating that the recorded IPSCs were mediated by activation of GABAA receptors. (H) Bath application of LY354740 (3 μM) inhibited the slope of the evoked field potentials recorded from layer III.
The above experiments were performed in the presence of bicuculline to exclude the contamination of GABAergic inputs. However, in physiological condition, mGluR II function concurrently with full GABAergic activity and it is unknown whether mGluR II exert any effects on GABAergic transmission although we have shown previously that activation of mGluR II has no effects on spontaneous IPSCs in the EC (Deng et al. 2010). We therefore initially measured the reversal potential of the evoked IPSCs induced by GABAA receptors with the same intracellular solution (Cs+-gluconate) but in the extracellular solution containing dl-APV (50 μM) and DNQX (10 μM) to block glutamatergic responses. The reversal potential for the IPSCs was measured to be −43.5 ± 1.8 mV (n = 5, Fig. 1E,F) in our recording condition. We then recorded the evoked IPSCs at −70 mV using the same intracellular and extracellular solution and tested whether activation of mGluR II modulates GABAergic transmission. However, application of LY354740 (3 μM) failed to change the amplitude of evoked IPSCs significantly (99 ± 3% of control, n = 7, P = 0.69, Fig. 1G) and subsequent application of bicuculline (10 μM) at the end of the experiments completely blocked the evoked IPSCs indicating that the recorded currents were mediated by GABAA receptors. These results collectively demonstrate that activation of mGluR II does not modulate GABAergic transmission, consistent with our previous results (Deng et al. 2010). Because we made a cut between layer III and layer V to prevent bicuculline-induced propagation of epileptic activity, one may wonder whether the traumatic effect of the cutting contributes to the depression induced by mGluR II. We therefore recorded the evoked field potentials in the absence of bicuculline and measured the slope of the evoked potentials to evaluate the effects of LY354740. No cut was made between layer III and layer V of the slices for this experiment. Under these circumstances, bath application of LY354740 (3 μM) still induced robust inhibition on the slope of the evoked potentials (13 ± 3% of control, n = 9, P < 0.001, Fig. 1H) demonstrating that mGluR II–induced depression of glutamatergic transmission is unlikely due to the blockade of GABAergic response or the potential traumatic effects of the neurons.
Presynaptic Mechanism of mGluR II–Induced Depression of Glutamatergic Transmission in the EC
We next tested whether mGluR II–induced depression of glutamatergic transmission is presynaptic or postsynaptic by performing the following experiments. First, we measured the CV of AMPA EPSCs before and after the application of LY354740 or DCG-IV because changes in presynaptic transmitter release are usually concomitant with alterations in CV (Malinow and Tsien 1990; McAllister and Stevens 2000). CV was significantly increased after application of LY354740 (control: 0.129 ± 0.019, LY354740: 0.322 ± 0.067, n = 6, P = 0.02, Fig. 2A) or DCG-IV (control: 0.121 ± 0.066, DCG-IV: 0.263 ± 0.186, n = 6, P = 0.003, data not shown). Second, we measured the PPR of AMPA EPSCs before and after the application of LY354740 because a change in presynaptic release probability usually accompanies with an alteration of PPR (Zucker and Regehr 2002). Application of LY354740 significantly increased the PPR (control: 0.549 ± 0.054, LY354740: 0.865 ± 0.035, n = 6, P = 0.001, Fig. 2B). Third, we recorded NMDA EPSCs on the basis that if LY354740-induced depression of AMPA EPSCs via a presynaptic mechanism, it should also reduce NMDA EPSCs. Bath application of LY354740 (3 μM) also inhibited the amplitudes of NMDA EPSCs (27 ± 4% of control, n = 5, P < 0.001, Fig. 2C). The CV of the NMDA EPSCs was also significantly increased after application of LY354740 (control: 0.068 ± 0.016, LY354740: 0.205 ± 0.042, n = 5, P = 0.006, Fig. 2D). These data together indicate that mGluR II–induced depression is mediated by a reduction of presynaptic glutamate release. Further evidence to support a presynaptic effect of mGluR II was that the input resistance of the recorded postsynaptic layer III pyramidal neurons was not significantly altered after application of LY354740 in our recording conditions (control: 409 ± 30 MΩ, LY354740: 393 ± 18 MΩ, n = 6, P = 0.35, Fig. 2E).
Figure 2.
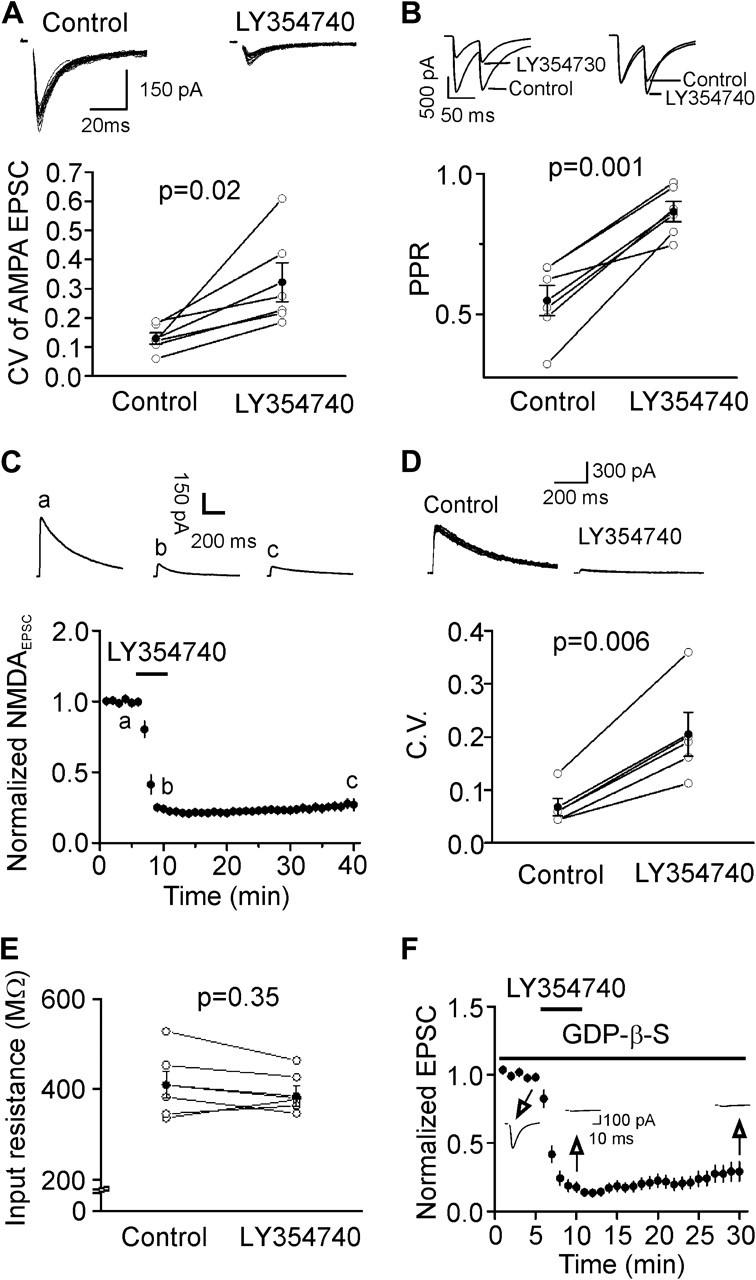
Presynaptic mechanism of mGluR II–mediated depression of glutamatergic transmission. (A) Application of LY354740 increased the CV of AMPA EPSCs. Upper panel shows 20 consecutive AMPA EPSCs recorded before and after the application of LY354740. Lower panel shows the calculated CVs from 6 cells (open circles) and their averages (solid circles). (B) Application of LY354740 increased PPR. Upper left, AMPA EPSCs evoked by 2 stimulations at an interval of 50 ms before and after the application of LY354740. Upper right, EPSCs recorded before and after the application of LY354740 were scaled to the first EPSC. Note that the second EPSC after the application of LY354740 is larger than control. Bottom, PPRs recorded from 6 cells (open circles) and their averages (solid circles). (C) Application of LY354740 inhibited NMDA EPSCs. Upper panel shows the average of 10 NMDA EPSCs recorded at different time points in the figure. (D) Application of LY354740 increased the CV of NMDA EPSCs. Upper panel shows 10 successive NMDA EPSCs recorded before and after the application of LY354740. Lower panel shows the calculated CVs from 5 cells (open circles) and their averages (solid circles). (E) Application of LY354740 failed to change the input resistance of layer III pyramidal neurons in the intracellular solution containing Cs+-gluconate. (F) Intracellular application of GDP-β-S via the recording pipettes did not significantly alter mGluR II–induced inhibition. Insets show the AMPA EPSCs recorded at different time points indicated in the figure.
Whereas mGluR II–induced inhibition of glutamatergic transmission is due to a reduction of presynaptic glutamate release, it is still possible that the mGluR II agonists activate postsynaptic mGluR II to generate retrograde messenger(s) to inhibit presynaptic glutamate release. If so, postsynaptic application of the G protein inactivator, GDP-β-S, via the recording pipettes should inhibit postsynaptic mGluR II and block LY354740-induced depression because mGluR II are G protein coupled. We included GDP-β-S (4 mM) in the recording pipettes and waited for >20 min after the formation of whole-cell configuration to permit the dialysis of GDP-β-S. Under these circumstances, bath application of LY354740 (3 μM) still induced robust inhibition of AMPA EPSCs (29 ± 7% of control, n = 5, P < 0.001, Fig. 2F) suggesting that it is unlikely that a postsynaptic retrograde messenger is involved.
NMDA Receptors and Presynaptic Stimulation Are Not Required for mGluR II–Mediated Inhibition of Glutamate Release
Activation of presynaptic NMDA receptors modulates glutamate release in the EC (Berretta and Jones 1996) and activation of mGluR II modifies NMDA receptor function (Tyszkiewicz et al. 2004). Furthermore, NMDA receptors are required for mGluR II–induced long-term depression (LTD) in rat prefrontal cortex (Otani et al. 2002). We therefore tested whether NMDA receptors are required for mGluR II–induced depression of glutamate release in the EC. In the presence of dl-AVP (100 μM), bath application of LY354740 (3 μM) still induced robust inhibition of AMPA EPSCs (34 ± 3% of control, n = 7, P < 0.001, Fig. 3A). We then tested if mGluR II–induced reduction in glutamate release relies on presynaptic stimulation. After recording basal AMPA EPSCs, presynaptic stimulation was stopped and LY354740 (3 μM) was applied without presynaptic stimulation for 5 min. LY354740 was then washed out before stimulation was resumed. Application of LY354740 in the absence of synaptic stimulation still induced comparable depression of AMPA EPSCs (27 ± 4% of control, n = 5, P < 0.001, Fig. 3B) indicating that presynaptic stimulation is not necessary for mGluR II–mediated reduction of glutamate release in the EC.
Figure 3.
NMDA receptors and presynaptic stimulation are not required for mGluR II–mediated inhibition of glutamate release. (A) Bath application of dl-APV (100 μM) failed to block mGluR II–induced depression of AMPA EPSCs. Upper panel shows the average of 10 EPSCs at different time points in the figure. (B) Presynaptic stimulation was not necessary for mGluR II–mediated depression of glutamate release. Basal AMPA EPSCs were recorded first, and synaptic stimulation was stopped. LY354740 (3 μM) was applied for 5 min, and bath was washed for additional 5 min without synaptic stimulation. AMPA EPSCs were recorded again with the same stimulation intensity. Note that robust inhibition was still induced under these circumstances.
Selective Reduction of Release Probability Is Responsible for mGluR II–Mediated Depression of Glutamate Release
Whereas our result that the PPR of AMPA EPSCs was increased after application of LY354740 suggests that the release probability (Pr) of glutamate is decreased, it is still possible that other mechanisms such as reductions of the quantal size (q) and/or the number of release sites (N) are involved. We therefore performed V–M analysis to determine the potential changes of these parameters after mGluR II–induced depression. We varied the release probability by using 3 different ratios of Ca2+/Mg2+ (4:1, 2:2, and 1:4 mM) in the extracellular solution. Under these conditions, the V–M relationship showed a parabola curve (Fig. 4A1). However, when we used LY354740 at 3 μM, the V–M relationship became linear (Fig. 4A2) indicating that the Pr was remarkably reduced at high concentrations of LY354740 as documented previously (Clements and Silver 2000). The linear relationship after application of high concentration of LY354740 prevented the determination of these parameters of synaptic transmission after mGluR II–induced depression of glutamate release (Clements and Silver 2000). We therefore performed a series of preliminary experiments and chose the concentration of 0.05 μM at which the V–M relationship after application of LY354740 was parabola for this experiment. Figure 4B1 illustrated the variance of 20 successive AMPA EPSCs when they were stabilized at each concentration before and after the application of LY354740 (0.05 μM). Figure 4B2 showed the peak amplitudes of 60 AMPA EPSCs recorded at the steady state of each concentration of Ca2+ before and after LY354740. As shown in Figure 4C1 (control) and Figure 4C2 (after LY354740), the V–M relationship obtained by varying [Ca2+]o is parabolic. The point of intersection of the parabolic curve and the abscissa was used to calculate the maximum EPSC (EPSCmax) for individual neurons. Pr at a given Ca2+ concentration was calculated as the mean EPSC divided by EPSCmax. For facilitation of comparisons across neurons, V–M relationships were normalized by dividing V and M by the EPSCmax estimated from the parabolic fit within individual neurons (Fig. 4D). Depression of glutamate release induced by activation of mGluR II was associated with significant reduction of Pr (Fig. 4E) without effects on q (control: 9.4 ± 2.7 pA, LY354740: 9.1 ± 2.5 pA, n = 5, P = 0.6) and N (control: 63.8 ± 21.4, LY354740: 61.2 ± 21.2, n = 5, P = 0.22) suggesting that mGluR II–induced inhibition of glutamatergic transmission is due mainly to a reduction of presynaptic glutamate release probability.
Figure 4.
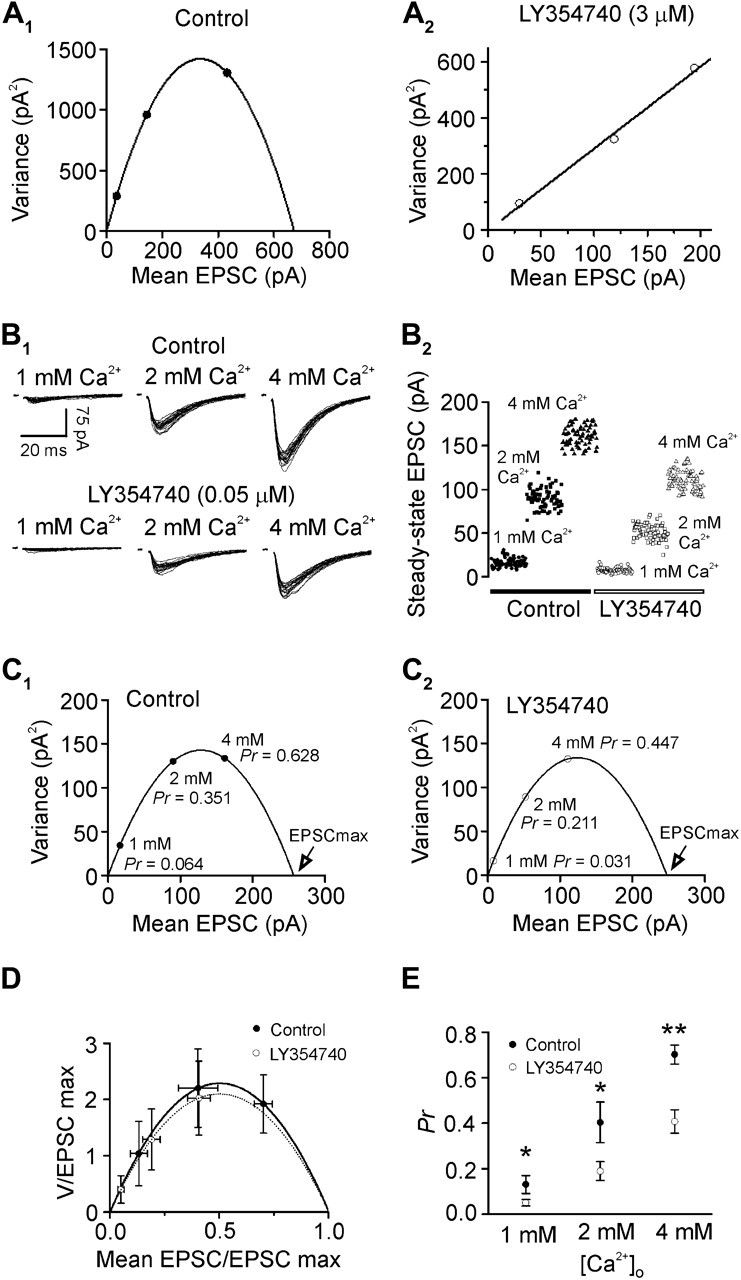
V–M analysis shows that mGluR II–mediated inhibition of glutamate release is associated with a reduction of Pr without effects on q and N. (A1) V–M plot for a layer III pyramidal neuron in control condition showed a parabolic relationship. (A2) V–M plot for the same layer III pyramidal neuron in A1 after application of LY354740 (3 μM) showed a linear relationship. (B1) Superimposed evoked EPSCs recorded at 1, 2, and 4 mM [Ca2+]o before (upper panel) and after (lower panel) the application of LY354740 (0.05 μM). (B2) Scatter plot of the EPSC peak amplitudes recorded at 1, 2, and 4 mM [Ca2+]o before (filled symbols) and after (open symbols) LY354740. (C1) V–M plot in control condition. Pr was calculated as the ratio of the mean EPSC amplitude at each concentration divided by the EPSCmax obtained by fitting the parabolic relationship. (C2) V–M plot after the application of LY354740 (0.05 μM) at the same synapse. Note that the Pr was reduced at each concentration of Ca2+ after application of LY354740 compared with control. (D) V and M values from each neuron were normalized by dividing each value by the predicted EPSCmax. Normalized curves were similar before and after the application of LY354740 indicating that q and N were not significantly changed by LY354740. (E) Pr values derived from the parabolic fits at different Ca2+ concentrations before and after LY354740. Note that LY354740 significantly attenuated Pr at each Ca2+ concentration (*P < 0.05; **P < 0.01).
An alternative approach of measuring q is to record mEPSCs in the presence of TTX. We therefore recorded mEPSCs and tested the effects of LY354740 on mEPSCs. Bath application of LY354740 (3 μM) significantly reduced the frequency of mEPSCs (57 ± 9% of control, n = 6, P = 0.004, Fig. 5A–C,E) with no effects on the amplitude of mEPSCs (98 ± 5% of control, n = 6, P = 0.73, Fig. 5D,F) confirming that LY354740 has no effects on q. Because mEPSCs are not action potential dependent, the result that the frequency of mEPSCs after application of LY354740 was significantly reduced suggests that activation of mGluR II interacts with glutamate release machinery. The inhibitory effect of LY354740 on the frequency of mEPSCs (57 ± 9% of control, n = 6) was significantly smaller than that of LY354740 on evoked EPSCs (19 ± 2% of control, n = 9, P < 0.001, unpaired t-test) suggesting that the action of mGluR II on glutamate release machinery contributes but is not solely responsible for mGluR II–induced inhibition of glutamate release.
Figure 5.
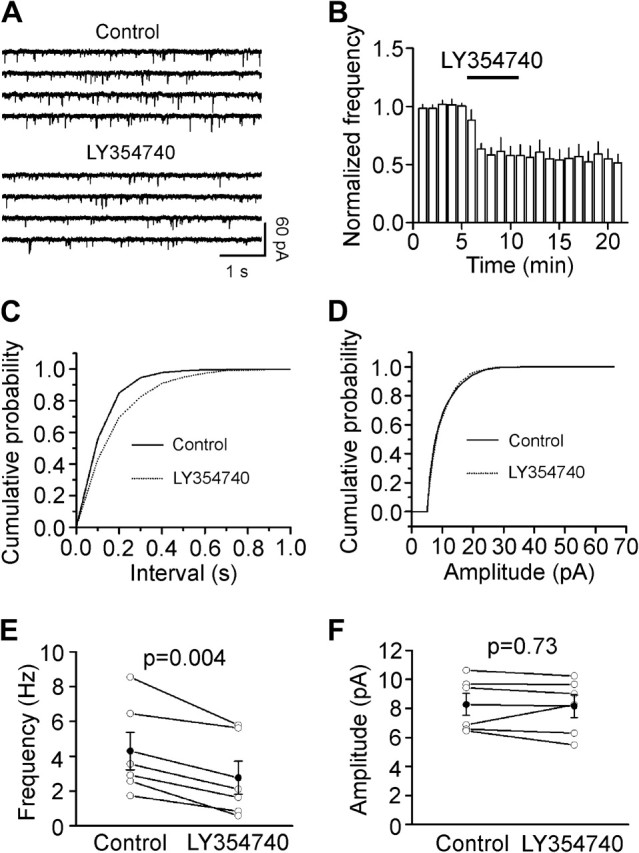
Activation of mGluR II decreases mEPSC frequency with no effects on mEPSC amplitudes. (A) mEPSCs recorded from a layer III pyramidal neuron in the presence of TTX (1 μM) before and after the application of LY354740 (3 μM). (B) Time course of the mEPSC frequency averaged from 6 cells. Numbers of mEPSCs at each min were normalized to that of mEPSCs in the 5 min prior to the application of LY354740. (C) Cumulative frequency distribution from a layer III pyramidal neuron before (solid) and after (dotted) the application of LY354740. Note that LY354740 increased the intervals of the mEPSC (decreased mEPSC frequency, P = 0.03, Kolmogorov–Smirnov test). (D) Cumulative amplitude distribution from the same cell before (solid) and after (dotted) the application of LY354740 (P = 0.67, Kolmogorov–Smirnov test). (E) Summarized data for LY354740-induced attenuation of mEPSC frequency (n = 6). (F) LY354740 failed to alter significantly mEPSC amplitudes (n = 6).
Inhibition of Presynaptic P/Q-type Ca2+ Channels Contributes to mGluR II–Induced Reduction of Glutamate Release
We next tested the roles of presynaptic Ca2+ channels in mGluR II–induced inhibition of glutamate release because G protein–coupled receptors inhibit Ca2+ channels (Herlitze et al. 1996; Ikeda 1996; Jarvis and Zamponi 2001). Bath application of the N-type Ca2+ channel blockers, ω-conotoxin GVIA (ω-CgTx-GVIA, 1 μM) irreversibly depressed AMPA EPSCs to 63 ± 5% of control (n = 5, P = 0.002, Fig. 6A) and subsequent application of LY354740 (3 μM) still induced robust inhibition (22 ± 5% of control, n = 5, P < 0.001, Fig. 6A). There were no statistically significant differences between the inhibition of AMPA EPSCs in control and that in the presence of ω-CgTx-GVIA (P = 0.43, unpaired t-test). Furthermore, bath application of ω-agatoxin IVA (ω-Aga-IVA, 200 nM) inhibited AMPA EPSCs to 43 ± 6% of control (n = 5, P < 0.001, Fig. 6B) and subsequent application of LY354740 (3 μM) induced a statistically smaller magnitude of depression (63 ± 5% of control, n = 5, Fig. 6B) compared with the extent of inhibition in control condition (P = 0.002, unpaired t-test). These data suggest that inhibition of P/Q-type Ca2+ channels contributes to mGluR II–induced depression of glutamate release. Together, we conclude that activation of mGluR II inhibits glutamate release via inhibitions of both the release machinery and the presynaptic P/Q-type Ca2+ channels.
Figure 6.
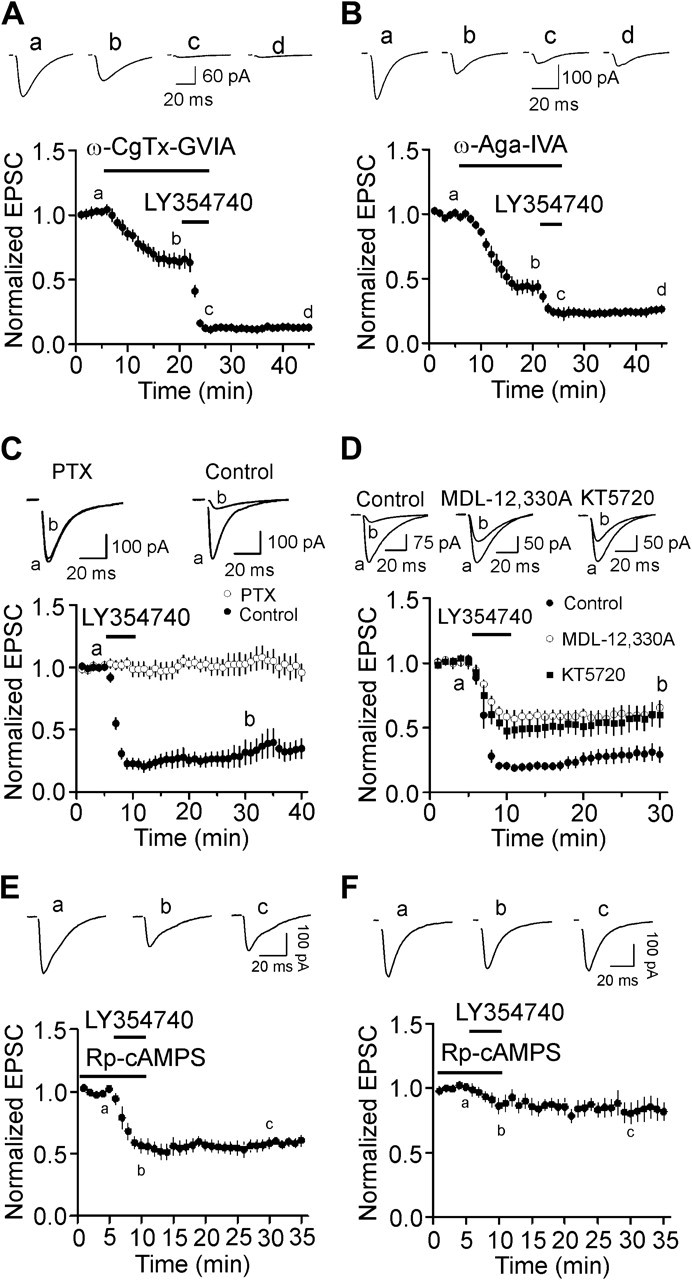
Presynaptic P/Q-type Ca2+ channels and PKA pathway are partially involved in mGluR II–mediated depression of glutamate release. (A) Bath application of the N-type Ca2+ channel blocker, ω-CgTx-GVIA (1 μM), inhibited AMPA EPSCs but did not significantly alter mGluR II–mediated depression of AMPA EPSCs (n = 5). Upper panel shows the averaged trace of 10 EPSCs at different time points in the figure. (B) Bath application of the P/Q-type Ca2+ channel blocker, ω-Aga-IVA (200 nM), inhibited AMPA EPSCs and significantly reduced the extent of mGluR II–induced depression (n = 5). Upper panel shows the averaged trace of 10 EPSCs at different time points in the figure. (C) Application of LY354740 failed to inhibit significantly AMPA EPSCs in slices pretreated with PTX but still induced robust inhibition of AMPA EPSCs in slices undergone the same fashion of treatment without PTX. Upper panel shows the averaged trace of 10 EPSCs at different time points in the figure. (D) Application of LY354740 induced a statistically smaller inhibition on AMPA EPSCs in slices treated with MDL-12,330A (50 μM) or KT5720 (1 μM). (E) Application of LY354740 induced a smaller magnitude of depression of AMPA EPSCs in the presence of Rp-cAMPS. Slices were pretreated with Rp-cAMPS (100 μM), and the same concentration of Rp-cAMPS was bath applied before and during the application of LY354740. (F) Simultaneous blockade of P/Q-type Ca2+ channels and inhibition of PKA remarkably decreased the level of mGluR II–mediated depression of AMPA EPSCs. Slices were pretreated with the irreversible P/Q-type Ca2+ channel blocker (ω-Aga-IVA, 200 nM) together with the specific PKA inhibitor (Rp-cAMPS, 100 μM) for ∼1 h. The same concentration of Rp-cAMPS was bath applied before and during the application of LY354740.
Signaling Mechanism
Because mGluR II are coupled to Gαi proteins (Conn and Pin 1997; Pin and Acher 2002), we tested whether the function of Gαi proteins is required for mGluR II–induced inhibition of glutamate release by applying the Gαi/o inhibitor, pertussis toxin (PTX). Slices were pretreated with PTX (5 μg/mL) for ∼10 h and application of LY354740 (3 μM) to the pretreated slices failed to inhibit AMPA EPSCs (103 ± 8% of control, n = 5, P = 0.75, Fig. 6C). However, application of LY354740 (3 μM) to the slices undergone the same fashion of treatment without PTX still induced robust inhibition (31 ± 9% of control, n = 5, P = 0.002, Fig. 6C). These data collectively indicate that Gαi proteins are required for mGluR II–induced inhibition of glutamate release.
Activation of Gαi proteins mediated by mGluR II results in depression of AC and subsequent inhibition of PKA (Conn and Pin 1997; Pin and Acher 2002). We next tested whether AC and PKA are involved in mGluR II–induced reduction of glutamate release. Slices were pretreated with the AC inhibitor, MDL-12,330A (50 μM) for ∼1 h, and the bath was continuously perfused with the same concentration of MDL-12,330A. Under these circumstances, application of LY354740 (3 μM) induced a smaller magnitude of depression (66 ± 5% of control, n = 6, P < 0.001 vs. the depression in control condition, Fig. 6D). We also tested the role of PKA in mGluR II–induced inhibition. Slices were pretreated with the selective PKA inhibitor, KT5720 (1 μM) for ∼1 h, and the bath was continuously perfused with the same concentration of KT5720. In the presence of KT5720, the extent of mGluR II–induced depression of AMPA EPSCs was also significantly reduced (60 ± 9% of control, n = 6, P < 0.001 vs. the extent of inhibition in control condition, Fig. 6D). We also used another specific PKA inhibitor, Rp-cAMPS. Slices were preincubated with Rp-cAMPS (100 μM) for ∼1 h, and the same concentration of Rp-cAMPS was bath applied before and during the application of LY354740 (3 μM). In this condition, the magnitude of mGluR II–induced depression was significantly reduced (57 ± 6% of control, n = 6, P < 0.001 vs. the inhibition in control condition, Fig. 6E). These results suggest that PKA pathway partially contributes to mGluR II–induced depression of glutamate release.
Our results demonstrate that mGluR II–induced inhibition of glutamate release involves 2 mechanisms: inhibition of presynaptic P/Q-type Ca2+ channels and direct interaction with the release machineries. Because PKA pathway is only partially required for mGluR II–mediated depression, it is unlikely that PKA is involved in both mechanisms. Accordingly, we tested which of the 2 mechanisms is related to the function of PKA. If PKA is not involved in mGluR II–induced inhibition of P/Q-type Ca2+ channels but responsible for mGluR II–mediated interaction with the release machinery, coapplication of ω-Aga-IVA and Rp-cAMPS would inhibit P/Q-type Ca2+ channels and the interaction with the release machinery, respectively, thereby obviating the contributions of P/Q-type Ca2+ channels and release machinery. In this situation, bath application of LY354740 would not induce inhibition or at least induces a smaller scale of inhibition on glutamate release. Conversely, if PKA is responsible for the mGluR II–induced inhibition of P/Q-type Ca2+ channels, coapplication of ω-Aga-IVA and Rp-cAMPS would act on the same target, P/Q-type Ca2+ channels, with no actions on the release machinery. Under these circumstances, bath application of LY354740 would still induce a comparable magnitude of depression. Because the inhibition of ω-Aga-IVA on P/Q-type Ca2+ channels is irreversible, we pretreated slices with Rp-cAMPS (100 μM) and ω-Aga-IVA (200 nM) for ∼1 h, and the same concentration of Rp-cAMPS was bath applied before and during the application of LY354740 (3 μM). In this condition, bath application of LY354740 (3 μM) induced a significantly smaller extent of inhibition (85 ± 5% of control, n = 8, P = 0.02 vs. the inhibition in the presence of ω-Aga-IVA alone, Fig. 6F). These results suggest that PKA is not involved in mGluR II–induced inhibition of P/Q-type Ca2+ channels but responsible for the mGluR II–mediated interaction with the release machinery. Consistent with our results, activation of PKA pathway has been shown to facilitate exocytosis processes (Trudeau et al. 1998; Evans and Morgan 2003; Nagy et al. 2004; Seino and Shibasaki 2005).
If mGluR II inhibits the release machinery by downregulation of PKA activity, functional upregulation of this pathway should increase mEPSC frequency. Application of forskolin (20 μM), an AC activator, significantly increased the frequency (164 ± 11% of control, n = 5, P = 0.004, Fig. 7A1) without effects on the amplitude (97 ± 3% of control, n = 5, P = 0.42) of mEPSCs. To test whether the function of PKA was involved in forskolin-induced enhancement of mEPSC frequency, slices were pretreated with KT5720 (1 μM), the selective PKA inhibitor, and the same concentration of KT5720 was continuously bath applied. In this situation, application of forskolin (20 μM) failed to alter the frequency (101 ± 14% of control, n = 8, P = 0.76, Fig. 7A2) and amplitude (106 ± 9% of control, n = 8, P = 0.51) of mEPSCs demonstrating that the effect of forskolin was mediated via activation of PKA. Similarly, bath application of 3-isobutyl-1-methylxanthine (IBMX) (500 μM), a phosphodiesterase inhibitor, also significantly enhanced the frequency (238 ± 41% of control, n = 5, P = 0.02, Fig. 7B1) with no effects on the amplitude (109 ± 8% of control, n = 5, P = 0.32) of mEPSCs and treatment of slices with KT5720 blocked the effects of IBMX (111 ± 10% of control, n = 5, P = 0.36, Fig. 7B2). We finally tested whether downregulation of PKA function changes mEPSCs. Bath application of KT5720 (1 μM) significantly inhibited the frequency (35 ± 9% of control, n = 7, P < 0.001, Fig. 7C) without effects on the amplitude (95 ± 6% of control, n = 7, P = 0.49) of mEPSCs. Likewise, bath application of MDL-12,330A (50 μM) reduced the frequency (33 ± 8% of control, n = 5, P = 0.001, Fig. 7D) without effects on the amplitude (116 ± 14% of control, n = 5, P = 0.323) of mEPSCs. These data together demonstrate that mEPSC frequency is under the tonic control of PKA function.
Figure 7.
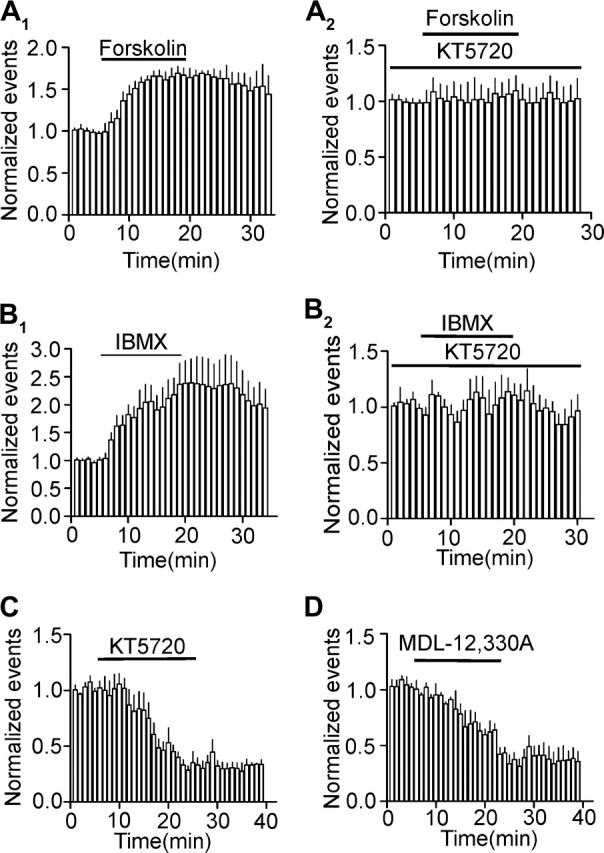
PKA-dependent tonic control of mEPSCs frequency. (A1) Bath application of forskolin (20 μM) enhanced mEPSC frequency. (A2) Pretreatment of slices with and continuous bath application of KT5720 (1 μM) counteracted forskolin-induced enhancement of mEPSC frequency. (B1) Bath application of IBMX (500 μM) significantly increased mEPSC frequency. (B2) Pretreatment of slices with and continuous bath application of KT5720 (1 μM) blocked IBMX-induced enhancement of mEPSC frequency. (C) Bath application of KT5720 (1 μM) reduced mEPSC frequency. (D) Bath application of MDL-12,330A (50 μM) inhibited mEPSC frequency.
Activation of Group II Metabotropic Glutamate Autoreceptors by Endogenously Released Glutamate Inhibits Glutamatergic Transmission
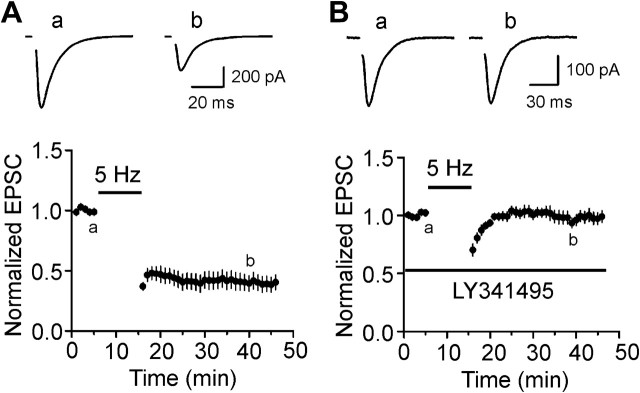
We finally tested the roles of endogenously released glutamate on mGluR II–induced inhibition of glutamatergic transmission. Whereas application of low-frequency stimulation (1 Hz) induces inhibition of glutamatergic transmission in the form of LTD which is either NMDA receptor dependent or mGluR dependent at individual synapses (Yokoi et al. 1996; Domenici et al. 1998; Tzounopoulos et al. 1998; Cho et al. 2000), application of this stimulation protocol in the EC induces NMDA receptor–dependent LTD (Solger et al. 2004; Deng and Lei 2007). Because the difference of the protocols to induce NMDA receptor–dependent and mGluR–dependent LTD is subtle (Cho et al. 2000), we tried another stimulation protocol (5 Hz for 10 min) to test the roles of mGluR II in glutamatergic transmission. Synaptic stimulation at this frequency has been shown to induce mGluR-dependent LTD (Oliet et al. 1997). The extracellular solution was supplemented with dl-APV (100 μM) and bicuculline (10 μM). Under these circumstances, application of the stimulation protocol (5 Hz for 10 min) inhibited AMPA EPSCs (43 ± 7% of control, n = 7, P < 0.001, Fig. 8A). The CV of the AMPA EPSCs was significantly increased after application of the stimulation protocol (control: 0.157 ± 0.032, after stimulation protocol: 0.308 ± 0.044, n = 7, P < 0.001) suggesting a presynaptic mechanism. In the presence of LY341495 (1 μM), application of the stimulation protocol did not induce the semipersistent depression of AMPA EPSCs (98 ± 6% of control, n = 8, P = 0.79, Fig. 8B). These data demonstrate that activation of mGluR II by endogenously released glutamate also inhibits glutamate release in the EC.
Figure 8.
Endogenously released glutamate induces depression of glutamatergic transmission via activation of mGluR II. (A) Presynaptic stimulation at 5 Hz for 10 min induced depression of AMPA EPSCs (n = 7). (B) Bath application of the mGluR II antagonist, LY341495, blocked the depression induced by endogenously released glutamate (n = 8).
Discussion
Although immunoreactivity for mGluR II has been detected in the EC, the function of mGluR II in synaptic transmission and plasticity in the EC has not been rigorously tested. We demonstrate that activation of mGluR II inhibits glutamatergic transmission without effects on GABAergic transmission onto layer III pyramidal neurons in the EC. The depression induced by activation of mGluR II is mediated by a reduction of presynaptic glutamate release but independent of NMDA receptors and presynaptic stimulation. V–M analysis indicates that mGluR II–mediated depression of glutamatergic transmission is due to a selective reduction of glutamate release probability with no alterations of quantal size and the number of release site. Inhibitions of presynaptic release machinery and P/Q-type Ca2+ channels are required for mGluR II–mediated depression of glutamate release. PKA pathway is only partially involved in mGluR II–induced depression of glutamate release possibly by acting on the release machinery downstream of the Ca2+ influx. We further demonstrate that activation of mGluR II by endogenously released glutamate also induces depression of glutamatergic transmission in the EC.
Whereas mGluR II–mediated inhibition of glutamatergic transmission in the form of LTD has been observed at the hippocampal mossy fiber–CA3 (Yokoi et al. 1996; Domenici et al. 1998; Tzounopoulos et al. 1998), medial perforant path–dentate gyrus (Huang et al. 1999), the prefrontal cortex (Otani et al. 2002), the perirhinal cortex (Cho et al. 2000), and amygdala (Lin et al. 2000) synapses, the locus of mGluR II–mediated inhibition of glutamatergic transmission is not consistent in these studies. Both presynaptic (Tzounopoulos et al. 1998; Kobayashi et al. 1999; Lin et al. 2000) and postsynaptic (Huang et al. 1999; Cho et al. 2000; Otani et al. 2002) mechanisms have been described for mGluR II–induced depression of glutamatergic transmission. Our results demonstrate that the location of mGluR II–mediated depression of glutamatergic transmission in the EC is presynaptic based on the following bodies of evidence. First, the CV of AMPA EPSCs was significantly increased after mGluR II–induced depression. Second, PPR was also significantly enhanced after application of LY354740. Third, mGluR II–mediated depression was observed when NMDA EPSCs were used as an indicator to assess synaptic transmission. Finally, when GDP-β-S was applied to the postsynaptic cells via the recording pipettes to inhibit the function of postsynaptic mGluR II, mGluR II–mediated depression was still observed.
Activation of mGluR II by glutamate initiates the exchange of GDP for GTP on the Gαi/o subunit, allowing the dissociation of the Gβγ subunit. Gαi/o then inhibits AC resulting in a decreased level of cAMP and depression of PKA (Conn and Pin 1997; Pin and Acher 2002) to mediate biological effects, whereas Gβγ directly interacts with intracellular effectors such as ion channels. Our data demonstrate that the function of Gαi/o is required for mGluR II–induced depression of glutamate release because pretreatment of slices with the selective Gαi/o protein inhibitor, PTX, counteracted mGluR II–induced inhibition of glutamate release. We have further demonstrated that activation of mGluR II induces depression of glutamate release via 2 distinct mechanisms including inhibition of presynaptic P/Q-type Ca2+ channels and direct interaction with presynaptic release machinery. Inhibition of AC–cAMP–PKA pathway only partially blocked mGluR II–mediated inhibition suggesting that this pathway is involved in only one mechanism. Furthermore, elevation of AC–cAMP–PKA pathway facilitated, whereas downregulation of this pathway depressed mEPSC frequency suggesting that mEPSC frequency is under tonic control of AC–cAMP–PKA pathway. Because mEPSCs represent an action potential- or Ca2+-independent process of release, these results suggest that activation of the AC–cAMP–PKA pathway directly interacts with the exocytotic machinery to facilitate glutamate release. Consistent with our results, AC–cAMP–PKA pathway has been shown to enhance exocytosis via a direct action on the secretory machinery in a variety of secretory cells (Trudeau et al. 1998; Evans and Morgan 2003; Nagy et al. 2004; Seino and Shibasaki 2005).
The second mechanism underlying mGluR II–induced reduction of glutamate release is the inhibition of presynaptic P/Q-type Ca2+ channels. Our results suggest that this process is not dependent on the AC–cAMP–PKA pathway. Consistent with our results, numerous studies have shown that inhibition of presynaptic Ca2+ channels by G protein–coupled receptors is mediated directly by Gβγ subunits (Herlitze et al. 1996; Ikeda 1996; Jarvis and Zamponi 2001).
In the present study, we focused on the effects of mGluR II on synaptic transmission and found that activation of mGluR II does not modulate GABAergic transmission but exerts powerful inhibition on glutamatergic transmission onto layer III pyramidal neurons. However, the effects of group I and group III mGluRs on synaptic transmission in the EC are more complicated. For example, activation of group I mGluRs facilitates the frequency and amplitude of spontaneous IPSCs but depresses the amplitudes of evoked IPSCs in the EC (Deng et al. 2010). Similarly, activation of group III mGluRs depresses the amplitudes of evoked EPSCs but increases the frequency of spontaneous EPSCs in layer V neurons of the EC (Woodhall et al. 2007) via interactions with PKA and PKC (Evans et al. 2001). Furthermore, activation of group III mGluRs suppresses spontaneous IPSCs in layer V neurons but not in layer II neurons (Woodhall et al. 2001; Deng et al. 2010). The discrepancy of the biological effects of mGluRs may be due to the selective distribution of mGluRs in different layers of the EC.
Whereas the EC is a key structure involved in the consolidation and recall of memories (Haist et al. 2001; Squire et al. 2004; Dolcos et al. 2005; Steffenach et al. 2005), and high density of mGluR II has been detected in the EC (Shigemoto et al. 1992; Ohishi et al. 1993a, 1993b; Fotuhi et al. 1994), the roles of mGluR II in memory have not been determined. Prolonged antagonism of mGluR II results in the appearance of spatial memory deficits (Altinbilek and Manahan-Vaughan 2009) demonstrating that mGluR II are involved in modulating memory. We have demonstrated that presynaptic stimulation at 5 Hz within the theta frequency (4–12 Hz) induces depression of glutamatergic transmission via activation of mGluR II. Theta oscillations are associated with memory (Buzsaki 2002, 2005; Hasselmo 2006) and are found in the EC (Alonso and Llinas 1989; Burton et al. 2008). Whether mGluR II–mediated depression of glutamate release plays a role in mGluR II–mediated modulation of memory still remains to be investigated in the future.
Funding
National Institutes of Mental Health (MH082881 to S.L.).
Acknowledgments
Conflict of Interest : None declared.
References
- Alexander GM, Godwin DW. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. 2006;71:1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Alonso A, Llinas RR. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. doi: 10.1038/342175a0. [DOI] [PubMed] [Google Scholar]
- Altinbilek B, Manahan-Vaughan D. A specific role for group II metabotropic glutamate receptors in hippocampal long-term depression and spatial memory. Neuroscience. 2009;158:149–158. doi: 10.1016/j.neuroscience.2008.07.045. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- Avoli M, D'Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-D-aspartate autoreceptors in the entorhinal cortex. Neuroscience. 1996;75:339–344. doi: 10.1016/0306-4522(96)00301-6. [DOI] [PubMed] [Google Scholar]
- Burton BG, Economo MN, Lee GJ, White JA. Development of theta rhythmicity in entorhinal stellate cells of the juvenile rat. J Neurophysiol. 2008;100:3144–3157. doi: 10.1152/jn.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- Clements JD. Variance-mean analysis: a simple and reliable approach for investigating synaptic transmission and modulation. J Neurosci Methods. 2003;130:115–125. doi: 10.1016/j.jneumeth.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Clements JD, Silver RA. Unveiling synaptic plasticity: a new graphical and analytical approach. Trends Neurosci. 2000;23:105–113. doi: 10.1016/s0166-2236(99)01520-9. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Bidirectional modulation of GABAergic transmission by cholecystokinin in hippocampal dentate gyrus granule cells of juvenile rats. J Physiol. 2006;572:425–442. doi: 10.1113/jphysiol.2005.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Lei S. Long-term depression in identified stellate neurons of juvenile rat entorhinal cortex. J Neurophysiol. 2007;97:727–737. doi: 10.1152/jn.01089.2006. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci. 2008;39:273–284. doi: 10.1016/j.mcn.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Porter JE, Shin HS, Lei S. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J Physiol. 2006;577:497–511. doi: 10.1113/jphysiol.2006.118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Poudel SK, Rojanathammanee L, Porter JE, Lei S. Serotonin inhibits neuronal excitability by activating two-pore domain K+ channels in the entorhinal cortex. Mol Pharmacol. 2007;72:208–218. doi: 10.1124/mol.107.034389. [DOI] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Lei S. Distinct modes of modulation of GABAergic transmission by Group I metabotropic glutamate receptors in rat entorhinal cortex. Hippocampus. 2010;20:980–993. doi: 10.1002/hipo.20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S. GABAB receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron. 2009;63:230–243. doi: 10.1016/j.neuron.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol. 1998a;398:49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998b;398:25–48. [PubMed] [Google Scholar]
- Domenici MR, Berretta N, Cherubini E. Two distinct forms of long-term depression coexist at the mossy fiber-CA3 synapse in the hippocampus during development. Proc Natl Acad Sci U S A. 1998;95:8310–8315. doi: 10.1073/pnas.95.14.8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Schwarcz R. Aminooxyacetic acid causes selective neuronal loss in layer III of the rat medial entorhinal cortex. Neurosci Lett. 1992;147:185–188. doi: 10.1016/0304-3940(92)90591-t. [DOI] [PubMed] [Google Scholar]
- Du F, Whetsell WO, Jr., Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- Evans DI, Jones RS, Woodhall G. Activation of presynaptic group III metabotropic receptors enhances glutamate release in rat entorhinal cortex. J Neurophysiol. 2000;83:2519–2525. doi: 10.1152/jn.2000.83.5.2519. [DOI] [PubMed] [Google Scholar]
- Evans DI, Jones RS, Woodhall G. Differential actions of PKA and PKC in the regulation of glutamate release by group III mGluRs in the entorhinal cortex. J Neurophysiol. 2001;85:571–579. doi: 10.1152/jn.2001.85.2.571. [DOI] [PubMed] [Google Scholar]
- Evans GJ, Morgan A. Regulation of the exocytotic machinery by cAMP-dependent protein kinase: implications for presynaptic plasticity. Biochem Soc Trans. 2003;31:824–827. doi: 10.1042/bst0310824. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Rozumek M. Limbic pathology in schizophrenia: the entorhinal region—a morphometric study. Biol Psychiatry. 1988;24:515–521. doi: 10.1016/0006-3223(88)90162-x. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Standaert DG, Testa CM, Penney JB, Jr., Young AB. Differential expression of metabotropic glutamate receptors in the hippocampus and entorhinal cortex of the rat. Brain Res Mol Brain Res. 1994;21:283–292. doi: 10.1016/0169-328x(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4:1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Huang L, Killbride J, Rowan MJ, Anwyl R. Activation of mGluRII induces LTD via activation of protein kinase A and protein kinase C in the dentate gyrus of the hippocampus in vitro. Neuropharmacology. 1999;38:73–83. doi: 10.1016/s0028-3908(98)00168-3. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Iserhot C, Gebhardt C, Schmitz D, Heinemann U. Glutamate transporters and metabotropic receptors regulate excitatory neurotransmission in the medial entorhinal cortex of the rat. Brain Res. 2004;1027:151–160. doi: 10.1016/j.brainres.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW. Interactions between presynaptic Ca2+ channels, cytoplasmic messengers and proteins of the synaptic vesicle release complex. Trends Pharmacol Sci. 2001;22:519–525. doi: 10.1016/s0165-6147(00)01800-9. [DOI] [PubMed] [Google Scholar]
- Joyal CC, Laakso MP, Tiihonen J, Syvalahti E, Vilkman H, Laakso A, Alakare B, Rakkolainen V, Salokangas RK, Hietala J. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry. 2002;51:1005–1007. doi: 10.1016/s0006-3223(01)01368-3. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21:9608–9618. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, Takahashi T. Calcium-dependent mechanisms involved in presynaptic long-term depression at the hippocampal mossy fibre-CA3 synapse. Eur J Neurosci. 1999;11:1633–1638. doi: 10.1046/j.1460-9568.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Kohler C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol. 1986;246:149–169. doi: 10.1002/cne.902460202. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer's disease. J Mol Neurosci. 2001;17:225–232. doi: 10.1385/jmn:17:2:225. [DOI] [PubMed] [Google Scholar]
- Lei S, Deng PY, Porter JE, Shin HS. Adrenergic facilitation of GABAergic transmission in rat entorhinal cortex. J Neurophysiol. 2007;98:2868–2877. doi: 10.1152/jn.00679.2007. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. GABAB receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol. 2003;546:439–453. doi: 10.1113/jphysiol.2002.034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Wang SJ, Luo MZ, Gean PW. Activation of group II metabotropic glutamate receptors induces long-term depression of synaptic transmission in the rat amygdala. J Neurosci. 2000;20:9017–9024. doi: 10.1523/JNEUROSCI.20-24-09017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:6173–6178. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Reim K, Matti U, Brose N, Binz T, Rettig J, Neher E, Sorensen JB. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–429. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993a;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993b;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Otani S, Daniel H, Takita M, Crepel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002;22:3434–3444. doi: 10.1523/JNEUROSCI.22-09-03434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Makoff A, Brown S, Rees S, Emson P. Localization of mGluR4 protein in the rat cerebral cortex and hippocampus. Neuroreport. 1997;8:3349–3354. doi: 10.1097/00001756-199710200-00031. [DOI] [PubMed] [Google Scholar]
- Pin JP, Acher F. The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. Curr Drug Targets CNS Neurol Disord. 2002;1:297–317. doi: 10.2174/1568007023339328. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Silver RA. Estimation of nonuniform quantal parameters with multiple-probability fluctuation analysis: theory, application and limitations. J Neurosci Methods. 2003;130:127–141. doi: 10.1016/j.jneumeth.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Solger J, Wozny C, Manahan-Vaughan D, Behr J. Distinct mechanisms of bidirectional activity-dependent synaptic plasticity in superficial and deep layers of rat entorhinal cortex. Eur J Neurosci. 2004;19:2003–2007. doi: 10.1111/j.1460-9568.2004.03292.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Fang Y, Haydon PG. Modulation of an early step in the secretory machinery in hippocampal nerve terminals. Proc Natl Acad Sci U S A. 1998;95:7163–7168. doi: 10.1073/pnas.95.12.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszkiewicz JP, Gu Z, Wang X, Cai X, Yan Z. Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurones of rat prefrontal cortex. J Physiol. 2004;554:765–777. doi: 10.1113/jphysiol.2003.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Janz R, Sudhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21:837–845. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus. 2003;13:943–952. doi: 10.1002/hipo.10144. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Evans DI, Jones RS. Activation of presynaptic group III metabotropic glutamate receptors depresses spontaneous inhibition in layer V of the rat entorhinal cortex. Neuroscience. 2001;105:71–78. doi: 10.1016/s0306-4522(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Woodhall GL, Ayman G, Jones RS. Differential control of two forms of glutamate release by group III metabotropic glutamate receptors at rat entorhinal synapses. Neuroscience. 2007;148:7–21. doi: 10.1016/j.neuroscience.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Deng PY, Rojanathammanee L, Yang C, Grisanti L, Permpoonputtana K, Weinshenker D, Doze VA, Porter JE, Lei S. Noradrenergic depression of neuronal excitability in the entorhinal cortex via activation of TREK-2 K+ channels. J Biol Chem. 2009;284:10980–10991. doi: 10.1074/jbc.M806760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Fransen E, Hasselmo ME. mGluR-dependent persistent firing in entorhinal cortex layer III neurons. Eur J Neurosci. 2008;28:1116–1126. doi: 10.1111/j.1460-9568.2008.06409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]