Abstract
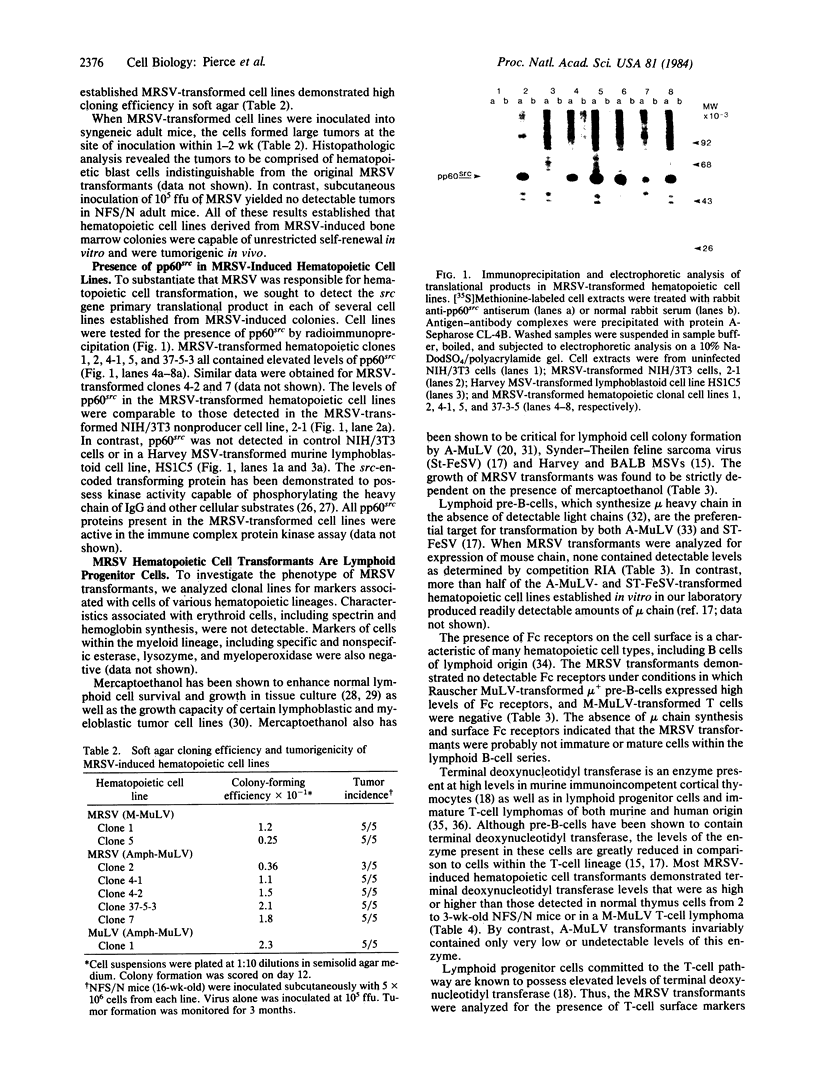
A recombinant murine retrovirus (MRSV) containing the src gene of avian Rous sarcoma virus (RSV) was shown to induce hematopoietic colonies in infected mouse bone marrow. MRSV-induced colony formation followed single-hit kinetics and required mercaptoethanol in the agar medium. Cells from the colonies induced by MRSV could be established as continuous cell lines that demonstrated unrestricted self-renewal in vitro and tumorigenicity in vivo. The transformants, all of which expressed high levels of the Rous sarcoma virus transforming protein, pp60src, appeared to be at an early stage in lymphoid cell differentiation. They lacked Fc receptors and detectable immunoglobulin mu heavy chain synthesis, markers normally associated with committed B cells. The majority of the MRSV-transformed cell lines contained high levels of terminal deoxynucleotidyl transferase, an enzyme present in lymphoid progenitor cells committed to the T-cell lineage. One cell line expressed Thy-1 antigen, but none expressed Lyt-1 and Lyt-2, markers of more differentiated T cells. These findings demonstrate that the src gene is capable of transforming cells of hematopoietic origin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Scolnick E. M. Construction and isolation of a transforming murine retrovirus containing the src gene of Rous sarcoma virus. J Virol. 1983 May;46(2):594–605. doi: 10.1128/jvi.46.2.594-605.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R., Goldschneider I., Bollum F. J. The distribution of terminal deoxynucleotidyl transferase (TdT) among subsets of thymocytes in the rat. J Immunol. 1976 Feb;116(2):462–468. [PubMed] [Google Scholar]
- Broome J. D., Jeng M. W. Growth stimulation of mouse leukemia cells by thiols and disulfides in vitro. J Natl Cancer Inst. 1972 Aug;49(2):579–581. [PubMed] [Google Scholar]
- Burrows P., LeJeune M., Kearney J. F. Evidence that murine pre-B cells synthesise mu heavy chains but no light chains. Nature. 1979 Aug 30;280(5725):838–840. doi: 10.1038/280838a0. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Lymphocytes as models for the study of mammalian cellular differentiation. Immunol Rev. 1977 Jan;33:105–124. doi: 10.1111/j.1600-065x.1977.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. Restoration of antibody-forming capacity in cultures of nonadherent spleen cells by mercaptoethanol. Science. 1972 Apr 7;176(4030):60–61. doi: 10.1126/science.176.4030.60. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Differential effects of transforming avian RNA tumor viruses on avian macrophages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3600–3604. doi: 10.1073/pnas.78.6.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHRUSSI B., TEMIN H. M. Infection of chick iris epithelium with the Rous sarcoma virus in vitro. Virology. 1960 Jul;11:547–552. doi: 10.1016/0042-6822(60)90099-4. [DOI] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Samarut J., Bouabdelli M., Blanchet J. P. Early precursors in the erythroid lineage are the specific target cells of avian erythroblastosis virus in vitro. Cell. 1980 Dec;22(3):683–691. doi: 10.1016/0092-8674(80)90544-9. [DOI] [PubMed] [Google Scholar]
- Hampe A., Laprevotte I., Galibert F., Fedele L. A., Sherr C. J. Nucleotide sequences of feline retroviral oncogenes (v-fes) provide evidence for a family of tyrosine-specific protein kinase genes. Cell. 1982 Oct;30(3):775–785. doi: 10.1016/0092-8674(82)90282-3. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Scolnick E. M. Harvey and Kirsten sarcoma viruses promote the growth and differentiation of erythroid precursor cells in vitro. Cell. 1981 Oct;26(1 Pt 1):91–97. doi: 10.1016/0092-8674(81)90036-2. [DOI] [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Radiommunoassays for the 70,000-molecular-weight glycoproteins of endogenous mouse type C viruses: viral antigen expression in normal mouse tissues and sera. J Virol. 1976 Jun;18(3):933–941. doi: 10.1128/jvi.18.3.933-941.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Kaighn M. E., Ebert J. D., Stott P. M. The susceptibility of differentiating muscle clones to Rous sarcoma virus. Proc Natl Acad Sci U S A. 1966 Jul;56(1):133–140. doi: 10.1073/pnas.56.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Davies A. J. The possible biological significance of Fc receptors on mammalian lymphocytes and tumor cells. Cell. 1974 Oct;3(2):105–112. doi: 10.1016/0092-8674(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Pragnell I. B. Retroviruses induce granulocyte-macrophage colony stimulating activity in fibroblasts. Nature. 1982 Oct 14;299(5884):638–640. doi: 10.1038/299638a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Warner N. L., Nossal G. J., Miller J. F., Shortman K., Rabellino E. Growth of B lymphocyte colonies in vitro from mouse lymphoid organs. Nature. 1975 Jun 19;255(5510):630–632. doi: 10.1038/255630a0. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Pepersack L., Lee J. C., McEwan R., Ihle J. N. Phenotypic heterogeneity of Moloney leukemia virus-induced T cel lymphomas. J Immunol. 1980 Jan;124(1):279–285. [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. BALB- and Harvey-murine sarcoma virus transformation of a novel lymphoid progenitor cell. J Exp Med. 1982 Sep 1;156(3):873–887. doi: 10.1084/jem.156.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. In vitro transformation of murine pre-B lymphoid cells by Snyder-Theilen feline sarcoma virus. J Virol. 1983 Jun;46(3):993–1002. doi: 10.1128/jvi.46.3.993-1002.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Dunn C. Y., Aaronson S. A. Different lymphoid cell targets by transformation by replication-competent Moloney and Rauscher mouse leukemia viruses. Cell. 1980 Mar;19(3):663–669. doi: 10.1016/s0092-8674(80)80043-2. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Characterization of temperature-sensitive mutants of murine leukemia virus. Virology. 1973 Jul;54(1):53–59. doi: 10.1016/0042-6822(73)90113-x. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Bruckenstein D. A., Bell A. P. Avian sarcoma virus gag precursor polypeptide is not processed in mammalian cells. J Virol. 1982 Nov;44(2):725–730. doi: 10.1128/jvi.44.2.725-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waneck G. L., Rosenberg N. Abelson leukemia virus induces lymphoid and erythroid colonies in infected fetal cell cultures. Cell. 1981 Oct;26(1 Pt 1):79–89. doi: 10.1016/0092-8674(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Abelson virus-infected cells can exhibit restricted in vitro growth and low oncogenic potential. J Virol. 1981 Nov;40(2):577–584. doi: 10.1128/jvi.40.2.577-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Ziegler S. F., Witte O. N. Progression of the transformed phenotype in clonal lines of Abelson virus-infected lymphocytes. Mol Cell Biol. 1983 Apr;3(4):596–604. doi: 10.1128/mcb.3.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]