Abstract
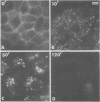
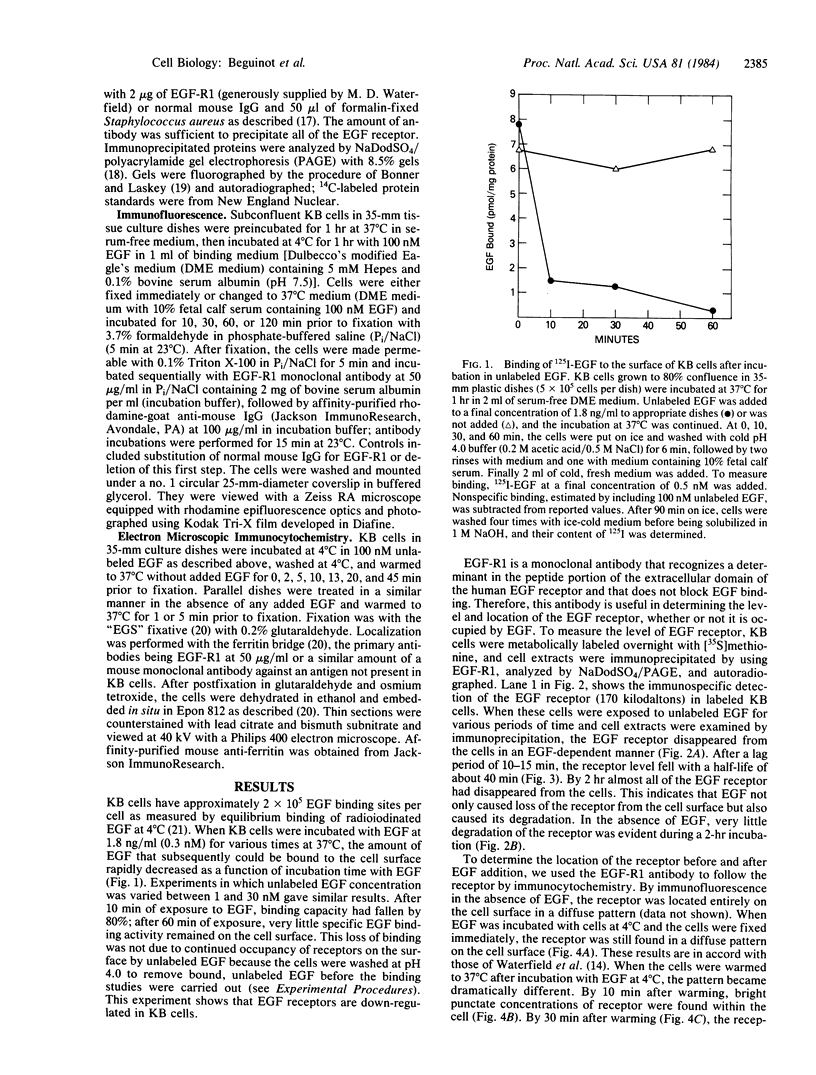
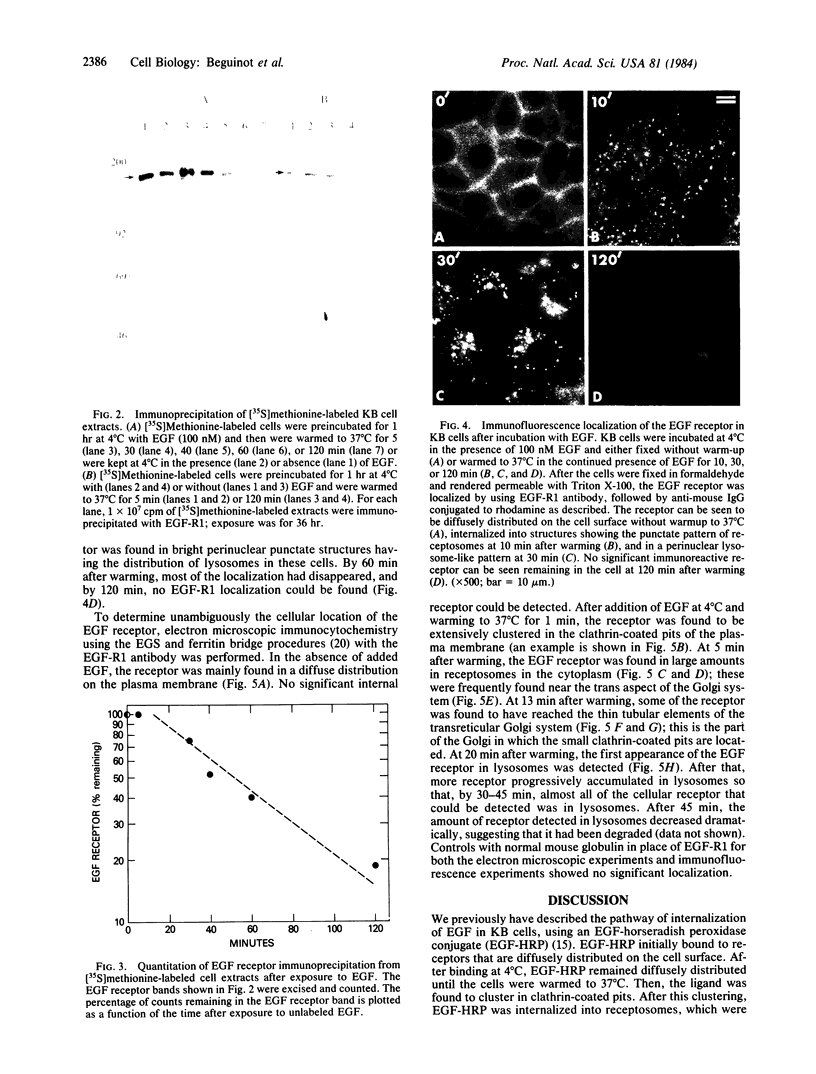
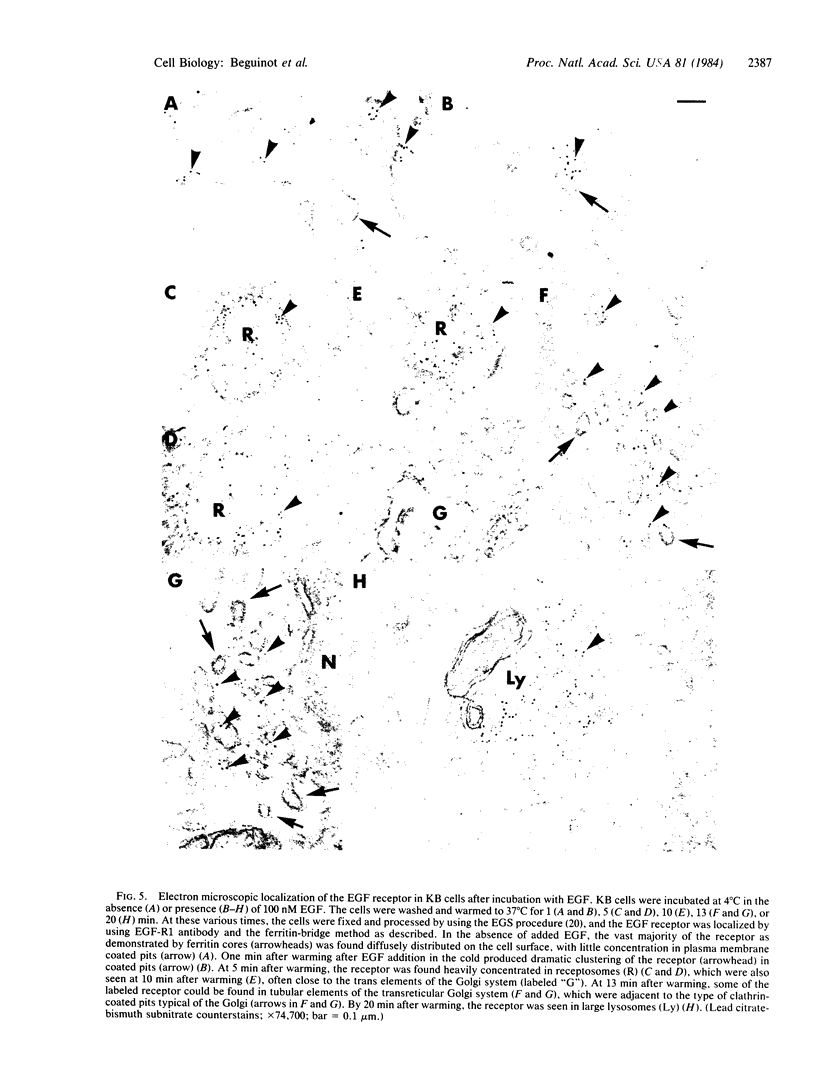
Using a monoclonal antibody to the human epidermal growth factor (EGF) receptor (EGF-R1), we have followed the metabolism of the receptor and the pathway of its internalization in KB cells after the addition of EGF. Measurement of surface binding of 125I-labeled EGF showed that about 80% of EGF binding activity disappeared from the plasma membrane after a 10-min exposure to EGF at 37 degrees C. Immunoprecipitation of the receptor from [35S]methionine-labeled cell extracts with EGF-R1 showed that EGF caused the receptor to be degraded with a half-life of 40 min. Immunofluorescence using EGF-R1 showed an EGF-dependent redistribution of the EGF receptor. In cells not exposed to EGF, almost all of the receptor was diffusely distributed on the cell surface. After EGF addition, the receptor was rapidly internalized, first appearing in small punctate organelles characteristic of receptosomes and then in larger perinuclear lysosome-like structures. By 120 min almost all of the immunoreactive EGF receptor had disappeared from the cells. Immunocytochemistry at the electron microscopic level confirmed these light microscopic findings. The diffusely distributed receptor on the cell surface first clustered into clathrin-coated pits in the presence of EGF, next was internalized into receptosomes, appeared transiently in transreticular Golgi elements, and finally was seen in lysosomes. This EGF-dependent down-regulation and degradation of the EGF receptor in KB cells provides a striking example of ligand-dependent clustering and internalization of a receptor, followed by degradation in lysosomes of both ligand and receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Stoscheck C. M., Preston Y. A., DeLarco J. E. Antibodies to the epidermal growth factor receptor block the biological activities of sarcoma growth factor. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5627–5630. doi: 10.1073/pnas.80.18.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Hanover J. A., Willingham M. C., Pastan I. Prelysosomal divergence of transferrin and epidermal growth factor during receptor-mediated endocytosis. Biochemistry. 1983 Nov 22;22(24):5667–5674. doi: 10.1021/bi00293a033. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Willingham M. C., Pastan I. Binding and internalization of 125I-alpha 2-macroglobulin by cultured fibroblasts. J Biol Chem. 1981 Apr 10;256(7):3454–3459. [PubMed] [Google Scholar]
- Fox C. F., Das M. Internalization and processing of the EGF receptor in the induction of DNA synthesis in cultured fibroblasts: the endocytic activation hypothesis. J Supramol Struct. 1979;10(2):199–214. doi: 10.1002/jss.400100210. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Kawamoto T., Sato J. D., Le A., Polikoff J., Sato G. H., Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. C., Willis R. A., Cuatrecasas P. Accumulation of epidermal growth factor within cells does not depend on receptor recycling. Biochem Biophys Res Commun. 1980 Dec 16;97(3):840–845. doi: 10.1016/0006-291x(80)91453-9. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Connolly D. T., Lane M. D. Synthesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11489–11496. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Richert N. D., Willingham M. C., Pastan I. Epidermal growth factor receptor. Characterization of a monoclonal antibody specific for the receptor of A431 cells. J Biol Chem. 1983 Jul 25;258(14):8902–8907. [PubMed] [Google Scholar]
- Schreiber A. B., Lax I., Yarden Y., Eshhar Z., Schlessinger J. Monoclonal antibodies against receptor for epidermal growth factor induce early and delayed effects of epidermal growth factor. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7535–7539. doi: 10.1073/pnas.78.12.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Mayes E. L., Stroobant P., Bennet P. L., Young S., Goodfellow P. N., Banting G. S., Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20(2):149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- Willingham M. C. Electron microscopic immunocytochemical localization of intracellular antigens in cultured cells: the EGS and ferritin bridge procedures. Histochem J. 1980 Jul;12(4):419–434. doi: 10.1007/BF01011958. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Haigler H. T., Fitzgerald D. J., Gallo M. G., Rutherford A. V., Pastan I. H. The morphologic pathway of binding and internalization of epidermal growth factor in cultured cells. Studies on A431, KB, and 3T3 cells, using multiple methods of labelling. Exp Cell Res. 1983 Jun;146(1):163–175. doi: 10.1016/0014-4827(83)90334-8. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Hanover J. A., Dickson R. B., Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):175–179. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. H. Transit of epidermal growth factor through coated pits of the Golgi system. J Cell Biol. 1982 Jul;94(1):207–212. doi: 10.1083/jcb.94.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]