Abstract
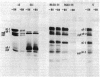
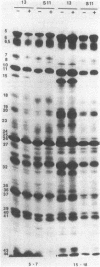
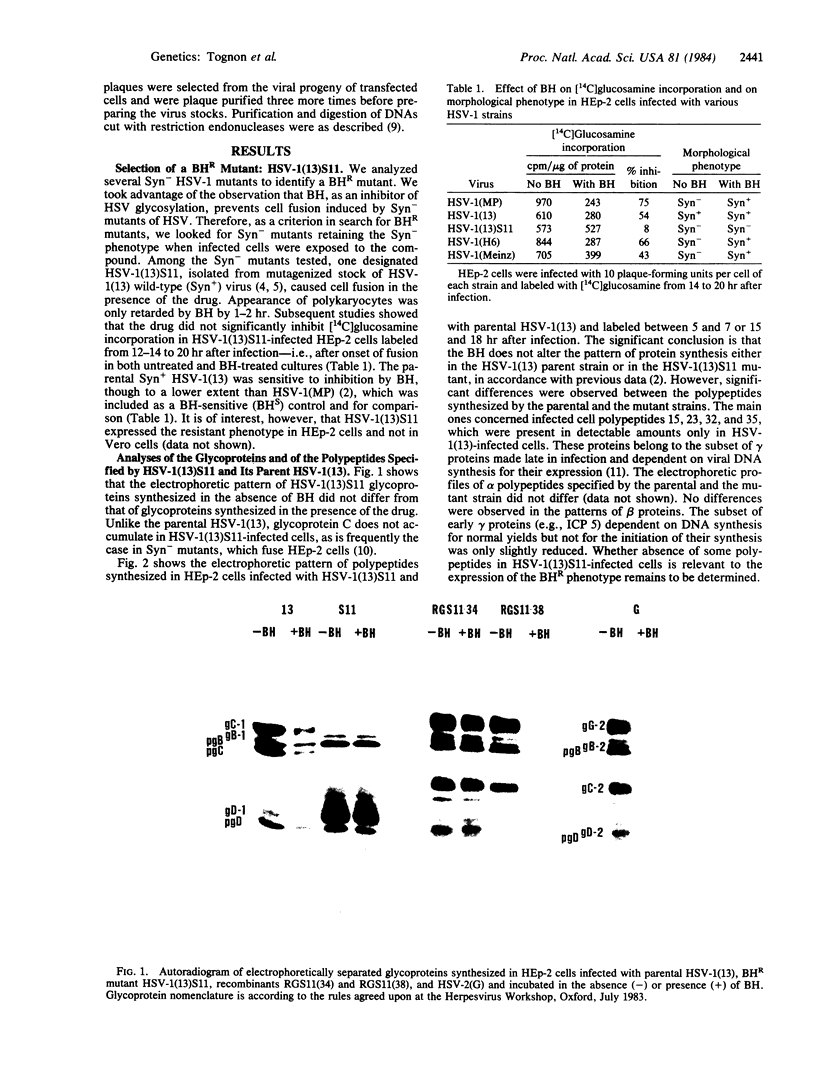
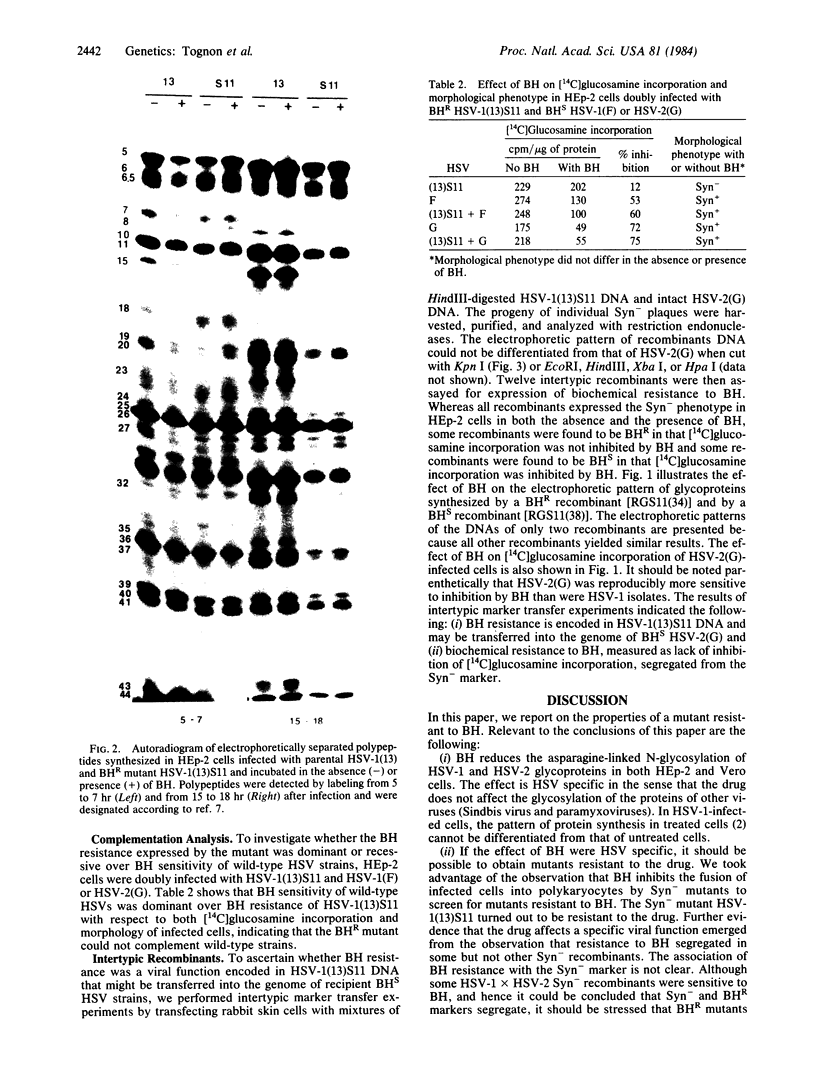
Benzhydrazone [BH; 1H-benz[f]indene-1,3(2H) -dionebis(amidinohydrazone)] significantly inhibits glycosylation of proteins, but only in cells infected with herpes simplex virus. We report on a herpes simplex virus type 1 (HSV-1) mutant resistant to BH. A syncytium-inducing mutant designated HSV-1(13)S11 was found to be biochemically resistant to BH in that [14C]glucosamine incorporation was not inhibited in infected HEp-2 cells exposed to the drug. Intertypic recombinants were obtained which showed that BH resistance is encoded in the DNA of the mutant virus and may be transferred into the genome of BH-sensitive HSV. In the recombinants the biochemical resistance marker segregated from the syncytial marker, suggesting that the two markers probably map in different loci. The BH-resistant mutant did not complement wild-type BH-sensitive HSV-1 and -2. Furthermore, resistance was apparent in HEp-2 but not in Vero cells. The paper discusses the hypothesis that inhibition of glycosylation of HSV proteins is the consequence of modification or selective transport of BH involving a HSV gene product.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campadelli-Fiume G., Sinibaldi-Vallebona P., Cavrini V., Mannini-Palenzona A. Selective inhibition of herpes simplex virus glycoprotein synthesis by a benz-amidinohydrazone derivative. Arch Virol. 1980;66(3):179–191. doi: 10.1007/BF01314732. [DOI] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffey M. L., Spear P. G. Alterations in glycoprotein gB specified by mutants and their partial revertants in herpes simplex virus type 1 and relationship to other mutant phenotypes. J Virol. 1980 Jul;35(1):114–128. doi: 10.1128/jvi.35.1.114-128.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J Virol. 1977 Feb;21(2):584–600. doi: 10.1128/jvi.21.2.584-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNYON W., KIT S. INHIBITION OF THYMIDINE KINASE FORMATION IN LM (TK-) CELLS SIMULTANEOUSLY INFECTED WITH VACCINIA AND A THYMIDINE KINASELESS VACCINIA MUTANT. Virology. 1965 Jun;26:374–377. doi: 10.1016/0042-6822(65)90287-4. [DOI] [PubMed] [Google Scholar]
- Manservigi R. Method for isolation and selection of temperature-sensitive mutants of herpes simplex virus. Appl Microbiol. 1974 Jun;27(6):1034–1040. doi: 10.1128/am.27.6.1034-1040.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Tognon M. Restriction endonuclease patterns of herpes simplex virus DNA: application to diagnosis and molecular epidemiology. Curr Top Microbiol Immunol. 1983;104:273–286. doi: 10.1007/978-3-642-68949-9_17. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Campadelli-Fiume G. Studies on benzhydrazone, a specific inhibitor of herpesvirus glycoprotein synthesis. Size distribution of glycopeptides and endo-beta-N-acetylglucosaminidase-H treatment. Arch Virol. 1981;70(4):331–343. doi: 10.1007/BF01320248. [DOI] [PubMed] [Google Scholar]
- Tognon M., Furlong D., Conley A. J., Roizman B. Molecular genetics of herpes simplex virus. V. Characterization of a mutant defective in ability to form plaques at low temperatures and in a viral fraction which prevents accumulation of coreless capsids at nuclear pores late in infection. J Virol. 1981 Dec;40(3):870–880. doi: 10.1128/jvi.40.3.870-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]