Abstract
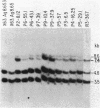
The frequencies of diverse rearrangements of variable (V)lambda to joining (J)lambda gene segments were examined by Southern blot hybridization in 30 murine B-cell lines, each producing an immunoglobulin lambda light chain of known subtype (lambda 1, lambda 2, or lambda 3). For 11 out of 12 lambda 1 chains, the rearrangement was V lambda 1----J lambda 1; for 9 out of 9 lambda 2 chains, it was V lambda 2----J lambda 2; and for 8 out of 9 lambda 3 chains, it was V lambda 1----J lambda 3. Similar results were obtained by considering the partial or complete sequences at the amino acid or cDNA level of 44 other lambda chains (24 previously described): for 43 of these chains the rearranged V-J gene segments were evidently V lambda 1-J lambda 1 for 28 lambda 1 chains, V lambda 2-J lambda 2 for 10 lambda 2 chains, and V lambda 1-J lambda 3 for 5 lambda 3 chains. Of the combined total of 74 chains there were 3 with unusual V lambda rearrangements, all involving the V lambda 2 gene segment: for 2 of these unusual chains, the encoding segments were V lambda 2-J lambda 1-C lambda 1 and for one they were V lambda 2-J lambda 3-C lambda 3. Thus, the results for all 74 lambda chains show that, in contrast to the apparently unrestricted V kappa----J kappa rearrangements for kappa chains, for each of the 3 murine lambda-chain subtypes V-J recombination is severely restricted: the V lambda gene segment expressed in lambda 1 and lambda 3 chains was nearly always V lambda 1 (95% and 93%, respectively), whereas in lambda 2 chains it was without exception V lambda 2 (19 out of 19 chains). Therefore V lambda-J lambda combinatorial variation is not a significant source of amino acid sequence diversity of lambda chains of inbred mice. If the order of the lambda gene segments is 5' V lambda 2-J lambda 2C lambda 2J lambda 4C lambda 4-V lambda 1-J lambda 3C lambda 3J lambda 1C lambda 1 3', as suggested previously and by the present findings, it appears that (i) when a V lambda gene segment rearranges in a developing B cell it ordinarily recombines with a J lambda gene segment in the nearest downstream (3') cluster of J lambda C lambda segments, and (ii) V lambda rearrangement to the upstream (5') cluster is very rare and possibly may not take place at all.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E. Amino acid sequences of two mouse immunoglobulin lambda chains. Proc Natl Acad Sci U S A. 1971 Mar;68(3):590–594. doi: 10.1073/pnas.68.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T., Steiner L. A., Eisen H. N. Identification of a third type of lambda light chain in mouse immunoglobulins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):569–573. doi: 10.1073/pnas.78.1.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Blomberg B., Geckeler W. R., Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972 Jul 14;177(4044):178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- Blomberg B., Tonegawa S. DNA sequences of the joining regions of mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):530–533. doi: 10.1073/pnas.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Traunecker A., Eisen H., Tonegawa S. Organization of four mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3765–3769. doi: 10.1073/pnas.78.6.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Schwartz R. C., Sonenshein G. E., Gefter M. L., Baltimore D. Dual expression of lambda genes in the MOPC-315 plasmacytoma. Nature. 1981 Mar 5;290(5801):65–67. doi: 10.1038/290065a0. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Cesari I. M., Weigert M. Mouse lambda-chain sequences. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2112–2116. doi: 10.1073/pnas.70.7.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Eustachio P., Bothwell A. L., Takaro T. K., Baltimore D., Ruddle F. H. Chromosomal location of structural genes encoding murine immunoglobulin lambda light chains. Genetics of murine lambda light chains. J Exp Med. 1981 Apr 1;153(4):793–800. doi: 10.1084/jem.153.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan E. S., Bradshaw R. A., Simms E. S., Eisen H. N. Amino acid sequence of the light chain of a mouse myeloma protein (MOPC-315). Biochemistry. 1973 Dec 18;12(26):5400–5416. [PubMed] [Google Scholar]
- Elliott B. W., Jr, Eisen H. N., Steiner L. A. Unusual association of V, J and C regions in a mouse immunoglobulin lambda chain. Nature. 1982 Oct 7;299(5883):559–561. doi: 10.1038/299559a0. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi-Kari T., Rajnavölgyi E., Takemori T., Jack R. S., Rajewsky K. The effect of light chain gene expression on the inheritance of an idiotype associated with primary anti-(4-hydroxy-3-nitrophenyl)acetyl(NP) antibodies. Eur J Immunol. 1979 Apr;9(4):324–331. doi: 10.1002/eji.1830090414. [DOI] [PubMed] [Google Scholar]
- Jack R. S., Imanishi-Kari T., Rajewsky K. Idiotypic analysis of the response of C57BL/6 mice to the (4-hydroxy-3-nitrophenyl)acetyl group. Eur J Immunol. 1977 Aug;7(8):559–565. doi: 10.1002/eji.1830070813. [DOI] [PubMed] [Google Scholar]
- Lenhard-Schuller R., Hohn B., Brack C., Hirama M., Tonegawa S. DNA clones containing mouse immunoglobulin kappa chain genes isolated by in vitro packaging into phage lambda coats. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4709–4713. doi: 10.1073/pnas.75.10.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Bothwell A., Storb U. Physical linkage of the constant region genes for immunoglobulins lambda I and lambda III. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3829–3833. doi: 10.1073/pnas.78.6.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Selsing E., Storb U. Structural alterations in J regions of mouse immunoglobulin lambda genes are associated with differential gene expression. Nature. 1982 Feb 4;295(5848):428–430. doi: 10.1038/295428a0. [DOI] [PubMed] [Google Scholar]
- Reth M., Imanishi-Kari T., Rajewsky K. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur J Immunol. 1979 Dec;9(12):1004–1013. doi: 10.1002/eji.1830091216. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Schilling J., Hansburg D., Davie J. M., Hood L. Analysis of the diversity of murine antibodies to dextran B1355: N-terminal amino acid sequences of heavy chains from serum antibody. J Immunol. 1979 Jul;123(1):384–388. [PubMed] [Google Scholar]
- Seidman J. G., Leder A., Edgell M. H., Polsky F., Tilghman S. M., Tiemeier D. C., Leder P. Multiple related immunoglobulin variable-region genes identified by cloning and sequence analysis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3881–3885. doi: 10.1073/pnas.75.8.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Brack C., Hozumi N., Schuller R. Cloning of an immunoglobulin variable region gene from mouse embryo. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3518–3522. doi: 10.1073/pnas.74.8.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M., Gatmaitan L., Loh E., Schilling J., Hood L. Rearrangement of genetic information may produce immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):785–790. doi: 10.1038/276785a0. [DOI] [PubMed] [Google Scholar]
- Weigert M., Perry R., Kelley D., Hunkapiller T., Schilling J., Hood L. The joining of V and J gene segments creates antibody diversity. Nature. 1980 Jan 31;283(5746):497–499. doi: 10.1038/283497a0. [DOI] [PubMed] [Google Scholar]
- White-Scharf M. E., Imanishi-Kari T. Characterization of the NPa idiotype through the analysis of monoclonal BALB/c anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies. Eur J Immunol. 1981 Nov;11(11):897–904. doi: 10.1002/eji.1830111109. [DOI] [PubMed] [Google Scholar]