Abstract
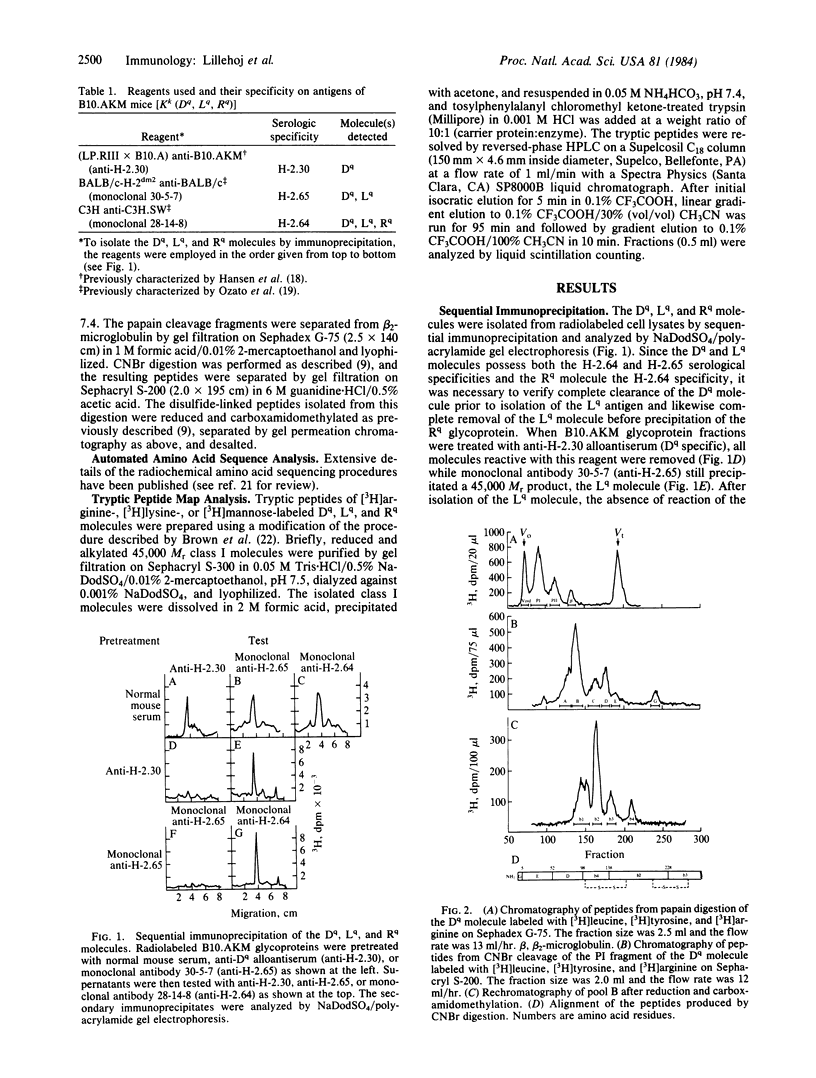
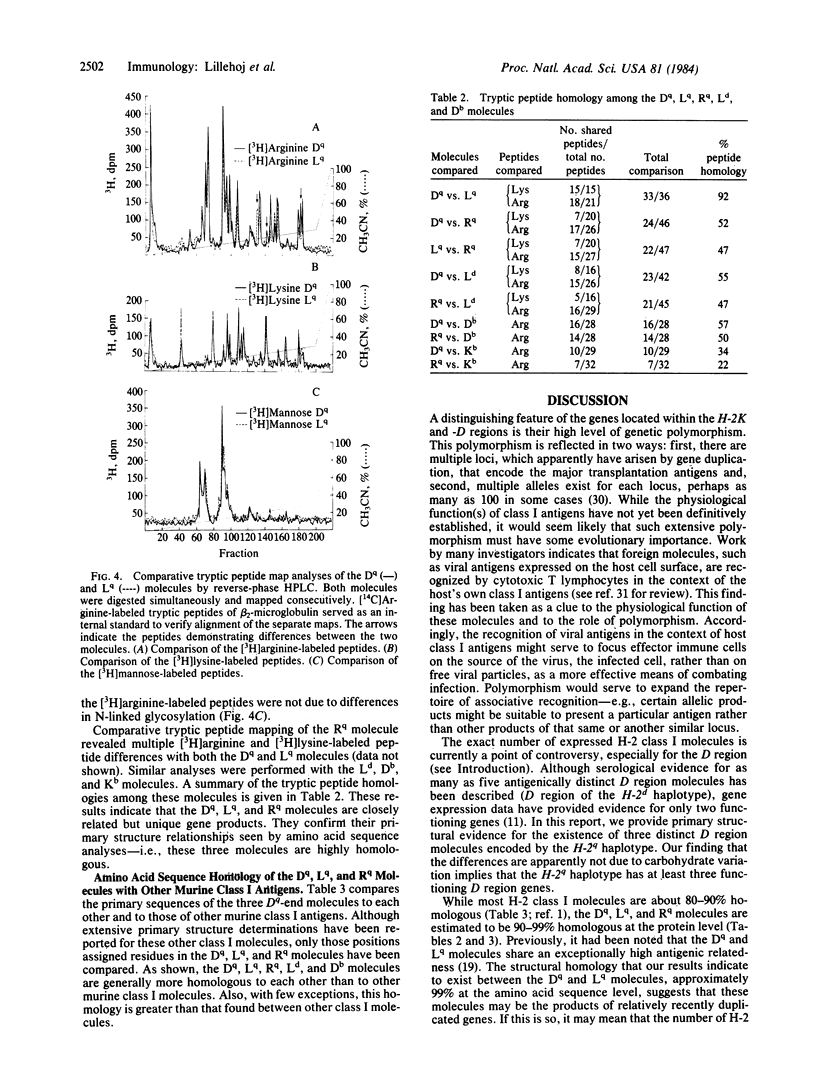
Class I gene products D, L, and R encoded by the D region of the H-2q haplotype were isolated by sequential immunoprecipitation from Nonidet P-40 extracts of B10.AKM murine spleen cells. Primary structural comparisons of these molecules were made by tryptic peptide map analyses and radiochemical amino acid sequence determinations. Partial NH2-terminal radiosequence analyses of the peptides generated by CNBr cleavage of these three molecules defined the Rq molecule as a gene product that is distinct from the Dq and Lq molecules. Comparative tryptic peptide map analyses of [3H]arginine-, [3H]lysine-, or [3H]mannose-labeled molecules were performed to determine if the Dq and Lq molecules could be distinguished. The two molecules were identical in their [3H]lysine-labeled peptides (17 in common) and [3H]mannose-labeled glycopeptides but could be differentiated by their [3H]arginine-labeled peptides (18 of 21 in common). These results suggest an amino acid sequence homology of 99% or greater between the Dq and Lq molecules. These data therefore provide structural evidence for three D region gene products and suggest that their antigenic differences are not due to post-translational glycosylation differences. Thus, the D region of the H-2q haplotype may provide an example of a relatively recent class I gene duplication, indicating that these genes are in a dynamic state of evolution through expansion or contraction of the gene pool.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. L., Kato K., Silver J., Nathenson S. G. Notable diversity in peptide composition of murine H-2K and H-2D alloantigens. Biochemistry. 1974 Jul 16;13(15):3174–3178. doi: 10.1021/bi00712a027. [DOI] [PubMed] [Google Scholar]
- Ciavarra R., Forman J. H-2L-restricted recognition of viral antigens In the H-2d haplotype, anti-vesicular stomatitis virus cytotoxic T cells are restricted solely by H-2L. J Exp Med. 1982 Sep 1;156(3):778–790. doi: 10.1084/jem.156.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J. Determination of protein primary structure by radiochemical techniques. J Immunol Methods. 1981;47(1):1–11. doi: 10.1016/0022-1759(81)90251-9. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Nairn R., Nathenson S. G., Sachs D. H., Hansen T. H. Primary structural studies of an H-2L molecule confirm that it is a unique gene product with homology to H-2K and H-2D antigens. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1134–1138. doi: 10.1073/pnas.77.2.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Cowan E. P., Cummings R. D., Schwartz B. D., Cullen S. E. Analysis of murine Ia antigen glycosylation by lectin affinity chromatography. I-Ak alpha chain subspecies and beta chains are each glycosylated differently. J Biol Chem. 1982 Oct 10;257(19):11241–11248. [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D., Nathenson S. G., Cherry M. The molecular basis of codominant expression of the histocompatibility-2 genetic region. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1394–1397. doi: 10.1073/pnas.69.6.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Démant P., Iványi D., Oudshoorn-Snoek M., Calafat J., Roos M. H. Molecular heterogeneity of H-2 antigens. Immunol Rev. 1981;60:5–22. doi: 10.1111/j.1600-065x.1981.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenow R. S., McMillan M., Nicolson M., Sher B. T., Eakle K., Davidson N., Hood L. Identification of the class I genes of the mouse major histocompatibility complex by DNA-mediated gene transfer. Nature. 1982 Nov 18;300(5889):231–237. doi: 10.1038/300231a0. [DOI] [PubMed] [Google Scholar]
- Götze D., Nadeau J., Wakeland E. K., Berry R. J., Bonhomme F., Egorov I. K., Hjorth J. P., Hoogstraal H., Vives J., Winking H. Histocompatibility-2 system in wild mice. X. Frequencies of H-2 and Ia antigens in wild mice from Europe and Africa. J Immunol. 1980 Jun;124(6):2675–2681. [PubMed] [Google Scholar]
- Hansen T. H., Ivanyi P., Levy R. B., Sachs D. H. Cross-reactivity among the products of three nonallelic H-2 loci, H-2Ld, H-2Dq, and H-2Kk. Transplantation. 1979 Oct;28(4):339–342. doi: 10.1097/00007890-197910000-00015. [DOI] [PubMed] [Google Scholar]
- Hansen T. H., Ozato K., Melino M. R., Coligan J. E., Kindt T. J., Jandinski J. J., Sachs D. H. Immunochemical evidence in two haplotypes for at least three D region-encoded molecules, D, L, and R. J Immunol. 1981 May;126(5):1713–1716. [PubMed] [Google Scholar]
- Hauptfeld V., Klein J. Molecular relationship between private and public H-2 antigens as determined by antigen redistribution method. J Exp Med. 1975 Aug 1;142(2):288–298. doi: 10.1084/jem.142.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iványi D., Démant P. One (H-2D2b) of the three Db region-controlled molecules (H-2D1b, H-2D2b, H-2Lb) is not detected in bm13 mutant. J Immunol. 1983 Sep;131(3):1080–1084. [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Klein J., Shreffler D. C. The H-2 model for the major histocompatibility systems. Transplant Rev. 1971;6:3–29. doi: 10.1111/j.1600-065x.1971.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Kvist S., Roberts L., Dobberstein B. Mouse histocompatibility genes: structure and organisation of a Kd gene. EMBO J. 1983;2(2):245–254. doi: 10.1002/j.1460-2075.1983.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. B., Hansen T. H. Functional studies of the products of H-2L locus. Immunogenetics. 1980;10(1):7–17. doi: 10.1007/BF01561548. [DOI] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E. Primary structure of the H-2Db alloantigen. II. Additional amino acid sequence information, localization of a third site of glycosylation and evidence for K and D region specific sequences. Immunogenetics. 1982;16(1):11–22. doi: 10.1007/BF00364438. [DOI] [PubMed] [Google Scholar]
- Margulies D. H., Evans G. A., Ozato K., Camerini-Otero R. D., Tanaka K., Appella E., Seidman J. G. Expression of H-2Dd and H-2Ld mouse major histocompatibility antigen genes in L cells after DNA-mediated gene transfer. J Immunol. 1983 Jan;130(1):463–470. [PubMed] [Google Scholar]
- Melino M. R., Nichols E. A., Strausser H. R., Hansen T. H. Characterization of H-2Db antigens implies haplotype differences in the number of H-2 molecules expressed. J Immunol. 1982 Jul;129(1):222–226. [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- Nairn R., Nathenson S. G., Coligan J. E. Amino acid sequence of cyanogen bromide fragment CN-C (residues 24-98) of the mouse histocompatibility antigen H-2Dd. A comparison of the amino-terminal 100 residues of H-2Dd, Dd, Kd, and Kb reveals discrete areas of diversity. Biochemistry. 1981 Aug 4;20(16):4739–4745. doi: 10.1021/bi00519a033. [DOI] [PubMed] [Google Scholar]
- Nairn R., Yamaga K., Nathenson S. G. Biochemistry of the gene products from murine MHC mutants. Annu Rev Genet. 1980;14:241–277. doi: 10.1146/annurev.ge.14.120180.001325. [DOI] [PubMed] [Google Scholar]
- Ozato K., Hansen T. H., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980 Dec;125(6):2473–2477. [PubMed] [Google Scholar]
- Rose S. M., Hansen T. H., Cullen S. E. Structural relation of murine "third locus" (H-2L) major histocompatibility antigens to the products of H-2K and H-2D loci. J Immunol. 1980 Nov;125(5):2044–2050. [PubMed] [Google Scholar]
- Snell G. D., Cherry M., Démant P. Evidence that H-2 private specificities can be arranged in two mutually exclusive systems possibly homologous with two subsystems of HL-A. Transplant Proc. 1971 Mar;3(1):183–186. [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]



