Abstract
Purpose.
Neuron-specific enolase (NSE) is a biomarker for neuronal stress. Leber's hereditary optic neuropathy (LHON) is a mitochondrial disease affecting retinal ganglion cells (RGC). These RGCs and their axons in the retinal nerve fiber layer (RNFL) and optic nerve head may show subclinical pathology in unaffected mutation carriers, or undergo cell death in affected patients. We hypothesize that increased levels of blood NSE may characterize LHON carriers as a biomarker of ongoing RGC stress.
Methods.
Serum was obtained from 74 members of a Brazilian pedigree with LHON carrying the homoplasmic 11778/ND4 mitochondrial DNA mutation. Classified by symptoms and psychophysical metrics, 46/74 patients were unaffected mutation “carriers,” 14/74 were “affected,” and 14/74 were “off-pedigree” controls. Serum NSE levels were determined by ELISA specific for the γ subunit of NSE.
Results.
Serum NSE concentrations in carriers (27.17 ± 39.82 μg/L) were significantly higher than affected (5.66 ± 4.19 μg/L; P = 0.050) and off-pedigree controls (6.20 ± 2.35 μg/L; P = 0.047). Of the 14/46 (30.4 %) carriers with significantly elevated NSE levels (mean = 75.8 ± 42.3 μg/L), 9/14 (64.3%) were male. Furthermore, NSE levels were nearly three times greater in asymptomatic male carriers (40.65 ± 51.21 μg/L) than in asymptomatic female carriers (15.85 ± 22.27 μg/L; P = 0.034).
Conclusions.
Serum NSE levels are higher in LHON carriers compared with affected and off-pedigree individuals. A subgroup of mostly male carriers had significantly elevated serum NSE levels. Thus, male carriers are at higher risk for LHON-related neuronal stress.
Asymptomatic carriers of LHON have higher serum NSE levels than affected and off-pedigree counterparts. Increased levels of serum NSE may be used as a biomarker to characterize RGC stress in LHON carriers.
Introduction
Leber's hereditary optic neuropathy (LHON) is a maternally inherited blinding disease caused by mitochondrial DNA (mtDNA) point mutations at positions 11,778/ND4, 3460/ND1, or 14,484/ND6 in ND subunit genes of complex I, which affect oxidative phosphorylation.1,2 In LHON pedigrees, these mutations are in most cases homoplasmic (100% mutant mtDNAs) in all maternally related individuals, but still only a few individuals become affected, thus displaying incomplete penetrance.3 This implicates that mtDNA mutations are necessary but not sufficient to trigger optic neuropathy.4 Both environmental and further genetic factors are believed to be relevant for disease expression. Tobacco smoking is a major environmental triggering factor for LHON,5 as well as the mtDNA genetic background influence on disease penetrance.6,7 However, still unknown nuclear genetic variants are also postulated to play a major modifying role in determining disease risk and it remains difficult to predict which individual carrying a LHON mutation will progress to blindness. Clinical onset of LHON is typically between 15 and 35 years of age and occurs more often in young men. Symptoms include rapid unilateral or bilateral loss of color vision and eventual loss of central vision due to the degeneration of retinal ganglion cells (RGCs) and axonal loss in the optic nerve.1,2 Such affected individuals go on to profound bilateral blindness and extensive optic atrophy.1,2
Neuron-specific enolase (NSE) is a glycolytic enzyme found in neuronal cells, specifically in mature neurons of the central nervous system (CNS). Neurons under stress of various types release NSE into the systemic circulation, making its assay a useful indicator of neuronal distress.8,9 NSE levels have been used to predict neurological outcome in traumatic brain injury.10 Retinal disease such as retinal detachment has also been shown to be sufficient to increase circulating serum NSE levels.11,12 Hence, we here hypothesized that NSE could be a useful biomarker for LHON, which is characterized by RGC distress. It might be highest in recently affected LHON patients during the acute phase with ongoing massive RGC loss, but high levels may also characterize LHON unaffected mutation carriers who have a full complement of RGC axons but may suffer subclinical disease. Furthermore, we predicted that LHON carriers with high NSE levels might be at the greatest risk for conversion to LHON affected.
Methods
Previous studies have characterized a very large Brazilian pedigree with LHON carrying the homoplasmic 11,778/ND4 mtDNA mutation.6,13–15 This study adhered to the tenets of the Declaration of Helsinki regarding the ethical principles for medical research involving human subjects and institutional review board approval was obtained. All patients gave informed consent and patient confidentiality was maintained throughout the study.
Serum was obtained from 74 individuals belonging to the family. Based on the pedigree and the clinical status, we classified 46/74 individuals as “unaffected mutation carriers” (from herein carriers) defined by the presence of homoplasmic 11,778/ND4 mutation with no visual symptoms.13–15 We also classified 14/74 individuals as “LHON affected” (affected) who carried the homoplasmic 11,778/ND4 mutation and suffered significant vision loss or blindness.13–15 Finally, we classified 14/74 individuals as “off pedigree” (controls) as being a family member not belonging to the maternal lineage, thus not carrying the 11,778/ND4 mutation and with no visual complaints.13–15 Among carriers, 21/46 were male and 25/46 female.
Blood samples were collected from each individual by venipuncture and placed in refrigerated storage. After allowing the samples to clot, serum was obtained by centrifugation at room temperature and immediately stored on dry ice at −20°C. Serum assays were performed within 6 months of collection.
Serum NSE concentration was determined by ELISA (Alpha Diagnostic International, San Antonio, TX). Each specimen measurement was performed in duplicate. Absorbance was measured on a micro-titer ELISA spectrophotometer (Benchmark Plus Microplate Spectrophotometer; Bio-Rad Laboratories, Irvine, CA) at 450 nm. The concentrations of samples and controls were determined based on a standard curve.
Results
Serum NSE levels significantly correlated with the state of LHON as defined by homoplasmic 11,778/ND4 mtDNA and the presence or absence of clinical symptoms of optic neuropathy. Asymptomatic carriers had on average 4-fold greater concentrations of serum NSE (27.17 ± 39.82 μg/L) than those with end-stage LHON (5.66 ± 4.19 μg/L, P = 0.050), as well as off-pedigree controls (6.20 ± 2.35 μg/L, P = 0.047) (Table 1). End-stage LHON patients have few RGCs left as demonstrated by their optic atrophy.1,2
Table 1. .
Comparing Average Serum NSE Concentration in Subclinical Carriers vs. Off-Pedigree Controls and Affected Patients
|
|
NSE |
P Value (Compared with Carriers) |
| Off pedigree | 6.20 ± 2.35 μg/L | 0.047 |
| Carriers | 27.17 ± 39.82 μg/L | |
| Affected | 5.89 ± 4.15 μg/L | 0.046 |
Among carriers, 70% (32/46) had NSE levels within the normal range (<20 μg/L), and 30% of carriers had NSE levels that were elevated, ranging from 24 to 147 μg/L. The average NSE concentration of individuals in this high-range group was 12 times greater than those in the normal range, suggesting differing levels of neuronal distress. Sixty-four percent of these high-range individuals were male. All patients (5/5) with NSE concentrations higher than 100 μg/L were male. In Figure 1, the blue line indicates an increase in NSE concentration as a function of the age of male patients and the red line, female patients. These data suggest that males may be experiencing more LHON-associated neuronal stress at earlier ages than females. Furthermore, NSE levels were nearly three times greater in asymptomatic male carriers (40.65 ± 51.21 μg/L) than in asymptomatic female carriers (15.85 ± 22.27 μg/L; P = 0.033). (Table 2) We also considered [NSE]/Age (years) as an index of risk. The NSE index was four times greater for asymptomatic males (1.98) than females (0.49).
Figure 1. .
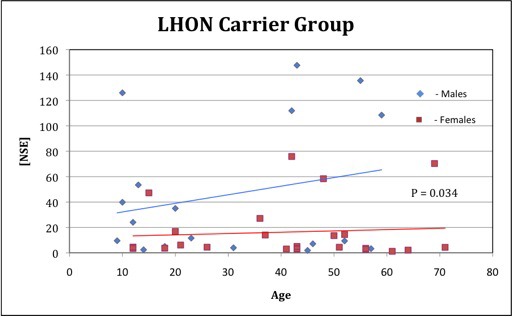
NSE concentration [NSE] and age in subclinical LHON carriers. Thirty-two (70%) of 46 carriers had [NSE] lower than 20 μg/L. Of carriers with [NSE] higher than 20 μg/L, 9 (64%) of 14 were males, and of those with [NSE] higher than 100 μg/L, all (5/5) were male.
Table 2. .
Comparing Average Serum NSE Concentration in Males vs. Females
|
|
NSE |
P Value |
| Carriers (n = 46) | 27.17 ± 39.82 μg/L | |
| Male carriers (n = 21) | 40.65 ± 51.21 μg/L | 0.033 |
| Female carriers (n = 25) | 15.85 ± 22.27 μg/L | |
| Off-pedigree controls (n = 14) | 6.20 ± 2.35 μg/L | |
| Male controls (n = 10) | 6.19 ± 2.13 μg/L | 0.966 |
| Female controls (n = 4) | 6.25 ± 3.22 μg/L |
Discussion
The present study shows that serum NSE levels are higher in unaffected mutation carriers as compared with controls, as well as LHON-affected patients. Affected LHON individuals with significant vision loss have NSE concentrations similar to off-pedigree controls, possibly because of a nearly complete loss of RGCs and their axons in the optic nerves.
NSE is a reliable bio-indicator of neuronal distress used to predict mortality or assess neurological status in CNS conditions like carbon monoxide poisoning or blunt head trauma.8,10 The normal range of NSE in serum of a healthy individual is 4 to 12 μg/L, with females ranging from 2.9 to 9.6 μg/L (mean = 5.3) and males with a slightly higher range of 3.4 to 11.7 μg/L (mean = 6.3).16 An individual with serum NSE higher than 12 μg/L is generally considered to be undergoing stress of neuronal and/or neuroendocrine cells.17 NSE is also found in the interphotoreceptor matrix of retinal photoreceptors,18 and it has been shown that rhegmatogenous retinal detachments have increased NSE levels in subretinal fluid, aqueous, and even circulating serum.12
In LHON, point mutations affecting complex I such as the 11,778/ND4 mutation lead to impaired oxidative phosphorylation.1,2 Bioenergetic failure, oxidative stress, glutamate toxicity, and increased propensity to undergo apoptosis as well as defective axonal transport dynamics have all been implicated as possible mechanisms contributing to abnormal function of mitochondria in RGCs.19,20 This altered mitochondrial function leads, by crossing a threshold, to sudden and massive retinal ganglion cell death and consequent loss of axons within the papillomacular bundle, which is characteristic of patients affected with LHON.1,2,21,22 However, many subclinical signs also characterize unaffected mutation carriers, in which RGC and axonal dysfunction, but not yet degeneration, are observed.1,2,15 Optical coherence tomography (OCT) imaging shows thickening of the retinal nerve fiber layer (RNFL) in asymptomatic carriers23 and during the acute phase of LHON,22 in relation to the stress and possibly swelling of RGC axons, preceding cell death. OCT has also shown the RNFL to be severely thinned at the end stage of LHON caused by the death of RGCs and depletion of their axons.24 Asymptomatic carriers also have subclinical color defects,25 decreased contrast sensitivity,26 pattern-reversal visual evoked potential (PRVEP) changes,27 and fundus abnormalities.15 We hypothesized that NSE may provide earlier risk detection for LHON conversion than structural changes detected on OCT and functional changes detected by PRVEP.
Elevated serum NSE was seen in at least 30% of LHON asymptomatic mutation carriers, all of whom, by definition, had normal visual acuity and did not manifest optic atrophy. A subgroup of carriers had significantly elevated serum NSE levels, most of them being males, in accordance with the greater risk for males than females to become affected. In particular, this study shows that male carriers had statistically higher NSE levels than female carriers, which is in keeping with their higher risk of conversion than the female counterparts. We considered [NSE]/Age (years) as an index of risk. Asymptomatic males had an average NSE index four times greater than asymptomatic females (P = 0.02) (Fig. 2). Thus, when considering NSE levels as a function of age, males appear to be at risk of conversion at an earlier age than females. There is some indication that on average the age of onset for females is later compared with males and conversion sometimes occurs after menopause.1,2 Estrogen may play a protective role in LHON, explaining these demographics.28
Figure 2. .
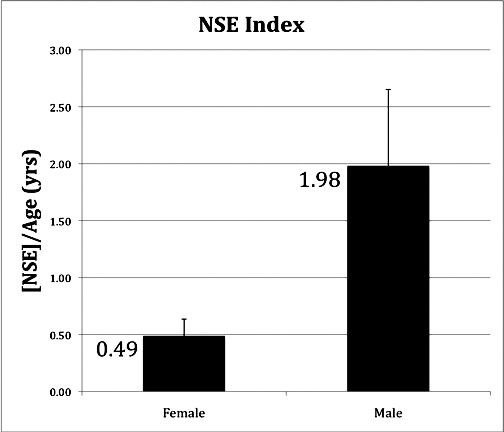
The average of NSE concentration divided by the patient's age in years. The NSE index is the patient's serum NSE concentration divided by his or her age in years. The average NSE index was four times greater in males (1.98) than in females (0.49) with P = 0.02. The SE was 0.15 and 0.67 in females and males, respectively.
Markedly increased serum NSE levels in asymptomatic carriers suggest that these individuals are undergoing neuronal distress, likely reflecting injury to RGCs and their axons not withstanding good visual function. High-risk individuals may be identified earlier by serum NSE levels rather than by structural abnormalities, such as seen with fundus examination or on OCT or functional losses as evidenced by loss of visual acuity, color, contrast, and central visual fields. Phosphorylated neurofilament heavy chain (pNF-H) has also been shown to be a reliable serum biomarker for neuronal injury, including LHON.29 However, this test may be both less sensitive and a later marker for this disease, as it is a measure of axonal death and not just stress. NSE levels would be expected to anticipate pNF-H in LHON and similar metabolic injury to neurons.
Serum NSE may be integrated in the clinical practice as a useful risk indicator for clinical conversion of LHON patients and thus may be a valuable asset in the early detection of disease progression. Such a biomarker may also allow for an object metric against which purported new treatments for LHON may be tested.30–32 Early detection may even allow for treatment before the full clinical conversion, which is manifested by a dramatic and rapid vision loss.
These studies were conducted in a very large single pedigree with the 11,778/ND4 mtDNA that has already been well characterized in many longitudinal studies.13–15 This minimizes the variability due to environmental factors, which are shared by the family, but may also enhance the influence of occasional nuclear or mtDNA genetic variants that may be specific to this family. The use of this one large family helps account for environmental variability, but does not account for genetic variability with respect to other LHON families with different genetic backgrounds, which may possibly influence the NSE levels. Hence, it would be useful to include other LHON families for NSE analysis before concluding on the advantages of this biomarker in LHON. Thus, to validate the current results, we look forward to longitudinal follow-up studies that examine the generality of possible NSE increases in other LHON pedigrees with different mtDNA haplogroups and primary mutations and nuclear genetic background, and, finally, to correlate increased serum NSE with conversion to a symptomatic state.
In conclusion, NSE has potential as a biomarker in LHON. New treatment protocols may benefit through the addition of serum NSE levels as a metric of RGC injury.
Footnotes
Supported by Research to Prevent Blindness, NEI Grant EY03040 (AAS), Struggling Within Leber's Fund, International Foundation for Optic Nerve Diseases, and VMR Consulting, Huntington Beach, California.
Disclosure: K.M. Yee, None; F.N. Ross-Cisneros, None; J.G. Lee, None; A. Bastos Da Rosa, None; S.R. Salomao, None; A. Berezovsky, None; R. Belfort Jr, None; F. Chicani, None; M. Moraes-Filho, None; J. Sebag, None; V. Carelli, None; A.A. Sadun, None
References
- 1.Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23:53–89 [DOI] [PubMed] [Google Scholar]
- 2.Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30:81–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell N, Mackey DA. Low-penetrance branches in matrilineal pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 1998;63:1220–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carelli V, Giordano C, d'Amati G. Pathogenic expression of homoplasmic mtDNA mutations needs a complex nuclear-mitochondrial interaction. Trends Genet. 2003;19:257–262 [DOI] [PubMed] [Google Scholar]
- 5.Kirkman MA, Yu-Wai-Man P, Korsten A, et al. Gene-environment interactions in Leber hereditary optic neuropathy. Brain. 2009;132:2317–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carelli V, Achilli A, Valentino ML, et al. Haplogroup effects and recombination of mitochondrial DNA: novel clues from the analysis of Leber hereditary optic neuropathy pedigrees. Am J Hum Genet. 2006;78:564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson G, Carelli V, Spruijt L, et al. Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background. Am J Hum Genet. 2007;81:228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yardan T, Cevik Y, Donderici O, et al. Elevated serum S100B protein and neuron-specific enolase levels in carbon monoxide poisoning. Am J Emerg Med. 2009;27:838–842 [DOI] [PubMed] [Google Scholar]
- 9.Johnsson P, Lundqvist C, Lindgren A, Ferencz I, Alling C, Ståhl E. Cerebral complications after cardiac surgery assessed by S-100 and NSE levels in blood. J Cardiothorac Vasc Anesth. 1995;9:694–699 [DOI] [PubMed] [Google Scholar]
- 10.Sogut O, Guloglu C, Orak M, et al. Trauma scores and neuron-specific enolase, cytokine and C-reactive protein levels as predictors of mortality in patients with blunt head trauma. J Int Med Res. 2010;38:1708–1720 [DOI] [PubMed] [Google Scholar]
- 11.Dunker S, Sadun AA, Sebag J. Neuron specific enolase in retinal detachment. Curr Eye Res. 2001;23:382–385 [DOI] [PubMed] [Google Scholar]
- 12.Quintyn JC, Pereira F, Hellot MF, Brasseur G, Coquerel A. Concentration of neuron-specific enolase and S100 protein in the subretinal fluid of rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2005;243:1167–1174 [DOI] [PubMed] [Google Scholar]
- 13.Sadun AA, Carelli V, Salomao S, et al. A very large Brazilian pedigree with 11778 Leber's hereditary optic neuropathy. Trans Am Ophthalmol Soc. 2002;100:169–180 [PMC free article] [PubMed] [Google Scholar]
- 14.Sadun AA, Carelli V, Salomao SR, et al. Extensive investigation of a large Brazilian pedigree of 11778/haplogroup J Leber hereditary optic neuropathy. Am J Ophthalmol. 2003;136:231–238 [DOI] [PubMed] [Google Scholar]
- 15.Sadun F, De Negri A, Carelli V, et al. Ophthalmologic findings in a large pedigree of 11778/Haplogroup J Leber hereditary optic neuropathy. Am J Ophthalmol. 2004;137:271–277 [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen LGM, Lober J, Carlsen NLT, et al. Serum neuron specific enolase (S-NSE) reference interval evaluation by time-resolved immunofluorometry compared with a radioimmunoassay. Clinica Chimica Acta. 1996;249:77–91 [DOI] [PubMed] [Google Scholar]
- 17.Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Ann Rev Neurosci. 1987;10:269–295 [DOI] [PubMed] [Google Scholar]
- 18.Li A, Lane WS, Johnson LV, Chader GJ, Tombran-Tink J. Neuron-specific enolase: a neuronal survival factor in the retinal extracellular matrix? J Neurosci. 1995;15:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carelli V, La Morgia C, Iommarini L, et al. Mitochondrial optic neuropathies: how two genomes may kill the same cell type? Biosci Rep. 2007;27:173–184 [DOI] [PubMed] [Google Scholar]
- 20.Carelli V, La Morgia C, Valentino ML, et al. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim Biophys Acta. 2009;1787:518–528 [DOI] [PubMed] [Google Scholar]
- 21.Sadun AA, Salomao SR, Berezovsky A, et al. Subclinical carriers and conversions in Leber hereditary optic neuropathy: a prospective psychophysical study. Trans Am Ophthalmol Soc. 2006;104:51–61 [PMC free article] [PubMed] [Google Scholar]
- 22.Barboni P, Carbonelli M, Savini G, et al. Natural history of Leber's hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117:623–627 [DOI] [PubMed] [Google Scholar]
- 23.Savini G, Barboni P, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber's hereditary optic neuropathy mutations. Ophthalmology. 2005;112:127–131 [DOI] [PubMed] [Google Scholar]
- 24.Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber's hereditary optic neuropathy. Ophthalmology. 2005;112:120–126 [DOI] [PubMed] [Google Scholar]
- 25.Ventura DF, Gualtieri M, Oliviera AGF, et al. Male prevalence of acquired color vision defects in asymptomatic carriers of Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2007;48:2362–2370 [DOI] [PubMed] [Google Scholar]
- 26.Ventura DF, Quiros P, Carelli V, et al. Chromatic and luminance contrast sensitivities in asymptomatic carriers from a large Brazilian pedigree of 11778 Leber hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2005;46:4809–4814 [DOI] [PubMed] [Google Scholar]
- 27.Sacai PY, Salomao SR, Carelli V, et al. Visual evoked potentials findings in non-affected subjects from a large Brazilian pedigree of 11778 Leber's hereditary optic neuropathy. Doc Ophthalmol. 2010;121:147–154 [DOI] [PubMed] [Google Scholar]
- 28.Giordano C, Montopoli M, Perli E, et al. Oestrogens ameliorate mitochondrial dysfunction in Leber's hereditary optic neuropathy. Brain. 2011;134:220–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guy J, Shaw G, Ross-Cisneros FN, et al. Phosphorylated neurofilament heavy chain is a marker of neurodegeneration in Leber hereditary optic neuropathy (LHON). Mol Vis. 2008;14:2443–50 [PMC free article] [PubMed] [Google Scholar]
- 30.Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain. 2011;134:2677–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carelli V, La Morgia C, Valentino ML, et al. Idebenone treatment in Leber's hereditary optic neuropathy. Brain. 2011;134:e188. [DOI] [PubMed] [Google Scholar]
- 32.Sadun AA, Chicani CF, Ross-Cisneros FN, et al. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch Neurol. 2012;69:331–338 [DOI] [PubMed] [Google Scholar]


