Abstract
Purpose.
We evaluated the effect of imposing negative and positive defocus simultaneously on the eye growth and refractive state of the common marmoset, a New World primate that compensates for either negative and positive defocus when they are imposed individually.
Methods.
Ten marmosets were reared with multizone contact lenses of alternating powers (−5 diopters [D]/+5 D), 50:50 ratio for average pupil of 2.80 mm over the right eye (experimental) and plano over the fellow eye (control) from 10 to 12 weeks. The effects on refraction (mean spherical equivalent [MSE]) and vitreous chamber depth (VC) were measured and compared to untreated, and −5 D and +5 D single vision contact lens-reared marmosets.
Results.
Over the course of the treatment, pupil diameters ranged from 2.26 to 2.76 mm, leading to 1.5 times greater exposure to negative than positive power zones. Despite this, at different intervals during treatment, treated eyes were on average relatively more hyperopic and smaller than controls (experimental-control [exp-con] mean MSE ± SE +1.44 ± 0.45 D, mean VC ± SE −0.05 ± 0.02 mm) and the effects were similar to those in marmosets raised on +5 D single vision contact lenses (exp-con mean MSE ± SE +1.62 ± 0.44 D. mean VC ± SE −0.06 ± 0.03 mm). Six weeks into treatment, the interocular growth rates in multizone animals were already lower than in −5 D-treated animals (multizone −1.0 ± 0.1 μm/day, −5 D +2.1 ± 0.9 μm/day) and did not change significantly throughout treatment.
Conclusions.
Imposing hyperopic and myopic defocus simultaneously using concentric contact lenses resulted in relatively smaller and less myopic eyes, despite treated eyes being exposed to a greater percentage of negative defocus. Exposing the retina to combined dioptric powers with multifocal lenses that include positive defocus might be an effective treatment to control myopia development or progression.
Introduction
The common marmoset (Callithrix jacchus) is a New World primate known to respond to visual form deprivationm as well as to imposed hyperopic and myopic defocus by adjusting the growth rate of its ocular components.1–7 The ability to compensate both types of defocus by increasing or decreasing eye growth supports the existence of a bidirectional visual compensatory mechanism, which is regulated locally and regional within the eye as shown by the ability of experimental animals to emmetropize after optic nerve section, and to compensate for full field as well as hemifield retinal defocus.8–16 The visual control of eye growth likely is linked to the dynamics of daily visual experience and the different dioptric demands to which the eyes are exposed. To understand how these might affect eye growth, it is necessary first to characterize the spatial and temporal integration of myopic and hyperopic defocus. Previous work done in chicks, mammals, and Old World primates suggests that alternating hyperopic defocus (a strong stimulus to induce myopia) with periods of myopic defocus or unrestricted vision can decelerate eye growth and prevent myopia.8,17,18 Additionally, when chick eyes are exposed to simultaneous hyperopic and myopic defocus using multifocal spectacle lenses19 or a combination of cross-cylinders and single vision lenses,20 the eye grows slower and adjusts focus toward the more myopic plane. This suggests that the retina does not just compensate for the average amount of blur, but it can differentiate the sign of competing defocus and guide the growth of the eye toward the plane of myopic defocus.19–22 Therefore, the compensation for simple myopic defocus in chicks, known to be weaker in mammals or primates,7,23–26 appears to be preferred when it is combined with hyperopic defocus.19,21,22 We examined the response to simultaneous myopic and hyperopic defocus imposed in the marmoset with multifocal contact lenses to test this hypothesis in a nonhuman primate model of eye growth and development of refractive state.
The effect that simultaneous defocus of opposite sign has on the growth of the primate eye has yet to be assessed. This question is relevant particularly since a recent small population clinical trial using bifocal lenses in children has reported a reduction in myopia progression,27 and it will help understand how the human eye integrates defocus signals of mixed nature. Manipulating defocus across the retina in humans with spectacles, contact lenses, or refractive surgery might be an effective management tool for myopia. We aimed to provide better control of lens centration under spontaneous eye movement by using contact lenses instead of spectacle lenses. Biometric parameters, such as anterior chamber depth (ACD), corneal curvature (CC), lens thickness (LT), choroidal thickness (CT), and retinal thickness (RT), which are suggested to change considerably during emmetropization,22,28–30 also were included in our analysis.
Methods
Ten juvenile marmosets (Callithrix jacchus) were reared with a custom-designed concentric multizone contact lens (Medlens Innovations Inc., Front Royal, VA) from an average age of 72 days (range 70–76 days) for 12 weeks (range 11.7–12 weeks). The lens was made of methafilcon A (55% water content, DK:17), and had a center zone of 1.5 mm, −5 diopters (D) followed by a +5 D ring 0.25 mm wide and four more rings 0.20 mm wide each, of alternating power. The total diameters of the contact lenses used were either 6.0 or 6.5 mm, all of which had an optical zone diameter of 3.6 mm and were plano to the periphery. The total diameters of the alternating −5 D/+5 D zones were designed to give a 50:50 ratio of plus versus minus surface area for the average juvenile marmoset pupil diameter of 2.8 mm, measured under ambient animal room illumination (∼700 lux).
Contact lenses were fit 0.10 mm over the flattest keratometry reading and an ophthalmoscope was used to assess the fit.5,7 Because of the anatomy of the ocular globe and eyelids of the marmosets, the fit of all these contact lenses resembles that of a scleral fit. A topical antibiotic was used following each measurement and no corneal complications were observed in any of the animals throughout treatment.
Pupil diameters in the experimental marmosets were measured without cycloplegia before and during treatment at animal room light levels (∼700 lux). Images were captured using NIH Image (NIH, Bethesda, MD), enhanced using Image J software filters (NIH), and the average of five measurements was obtained per eye.
The animals wore the multizone lens on the right eye (experimental eye) and a plano lens on the fellow eye (control eye) for an average of 9 hours light/15 hours dark cycle following an established protocol for contact lens rearing in marmosets.5,7 Untreated (N = 25), single-vision positive (N = 20, OD +5 D, OS plano) and negative contact lens-reared marmosets (N = 16, OD −5 D, OS plano) from earlier studies7 were used as age-matched controls. All animal care, treatment, and experimental protocols were approved by the SUNY College of Optometry Institutional Animal Care and Use Committee, and conformed with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Ocular biometry and refractive state were measured twice during pretreatment (3–4 weeks before lens rearing, and immediately before lens rearing) and four times during lens rearing (T1 four weeks, T2 eight weeks, T3 ten weeks, and T4 twelve weeks into treatment). To gain insight into the temporal characteristics of multifocal defocus, growth rates were calculated before treatment (pretreatment) and at three time intervals during treatment: early (4–6 weeks into treatment), mid (6–8 weeks into treatment), and late (8–12 weeks into treatment).
Refraction, keratometry, and ultrasound biometry were performed as described in a previously established protocol 30 minutes after the instillation of 2 drops of 1% cyclopentolate and measurements were completed within two hours.3,7,31 On-axis refraction was given as the average of retinoscopy and Hartinger Coincidence Refractometer (Carl Zeiss, Oberkochen, Germany), used routinely in the past in our lab.3,7,31
High frequency A-scan ultrasound (25 MHz, Panametrics; NDT, Ltd, Waltham, MA) was used to measure ACD, LT, vitreous chamber depth (VC), CT, and RT as performed in earlier studies.3,4,7,31,32
Statistics
The statistical analysis was performed using Stata (College Station, TX). Paired Student's t-test was used to evaluate the differences between treated and fellow eyes at each time point, while one-way ANOVA examined the differences between treatment groups. Pearson's linear correlation was used to evaluate the relationships between interocular differences in refraction and ultrasound biometry.
Results
The average marmoset pupil diameter during treatment was 2.52 mm (range 2.26–2.76 mm), which resulted in 1.78 times greater exposure to the negative than to the positive power zone. The ratio of negative-to-positive contact lens surface area that corresponded to the pupils measured during treatment ranged from 65% negative-to-35% positive for a pupil diameter of 2.26 mm, to 52% negative-to-48% positive for a pupil of 2.76 mm, all of which resulted in greater overall exposure to hyperopic defocus. Since it was not possible to measure the effective refractive state through the multizone contact lenses, 19 additional marmosets were refracted wearing plano lenses to assess the potential effects that contact lens fit and centration might have had on the final effective refractive state (Table 1). The refractive changes induced by plano lenses at 0, 20, and 40 degrees on the nasal retina, as well as at 20 on the temporal retina were not significant. There was, however, a significant overall effect toward increased hyperopia (mean change ± SE +1.22 ± 0.33 D) at 40 degrees on the temporal retina (nasal visual field).
Table 1. .
Refractive Effect of Plano Lenses (Ave ± SE) on 19 Marmosets
|
Angle |
No Lens (Av ± SE) |
with Plano Lens (Av ± SE) |
Difference (with Lens - No Lens) (Av ± SE) |
P Value |
| −40° | 0.21 ± 0.47 | 1.42 ± 0.31 | 1.22 ± 0.33 | 0.002 |
| −20° | 0.55 ± 0.50 | 0.59 ± 0.34 | 0.04 ± 0.36 | 0.91 |
| 0° | 1.00 ± 0.45 | 0.54 ± 0.34 | −0.45 ± 0.40 | 0.28 |
| +20° | 0.65 ± 0.35 | 0.45 ± 0.35 | −0.20 ± 0.35 | 0.58 |
| +40° | 0.59 ± 0.37 | 0.58 ± 0.40 | 0.02 ± 0.38 | 0.72 |
Negative values of the angles represent the temporal retina (nasal visual field), whereas positive values of the angles represent the nasal retina (temporal visual field).
Biometric and refractive data were obtained pretreatment at 42 to 50 days of age, at baseline (B; 70–76 days old) and at four consecutive time points during the 12 weeks of treatment (T1 at 98–105, T2 at 126–133, T3 at 140–147, and T4 at 153–161 days old). At the beginning of treatment there were no differences in vitreous chamber depth or refraction (mean spherical equivalent [MSE]) between experimental (exp) and contralateral (con) eyes (exp VC 5.83 ± 0.06 mm, control VC 5.84 ± 0.07 mm, P = 0.11; exp MSE mean ± SE +0.65 ± 0.51 D, control MSE mean ± SE +0.08 ± 0.76 D, P = 0.54). The overall effective axial myopic and hyperopic defocus on treated eyes was +5.65 D and −4.35 D, respectively.
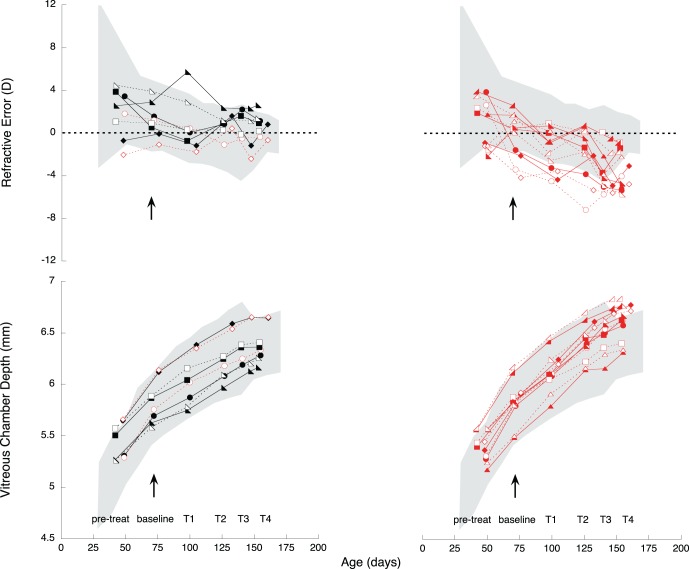
Figure 1 shows refractive and vitreous chamber depth data from treated and contralateral eyes (solid and empty symbols, respectively). The animals that were myopic at the end of treatment are indicated in red and the ones that were hyperopic in black. At baseline, three animals were myopic in both eyes (exp MSE mean ± SE −1.24 ± 0.0.60 D, control MSE mean ± SE −2.88 ± 0.92 D, Fig. 1) and three more had myopia in both eyes by the end of treatment, meaning that six of 10 animals were myopic in both eyes when treatment concluded (myopic treated eye MSE mean ± SE −3.39 ± 0.78 D, myopic fellow eye MSE mean ± SE −3.21 ± 0.78 D, Fig. 1), and eight were myopic on their contralateral fellow eyes (average of eight myopic contralateral eyes MSE mean ± SE −2.53 ± 0.72 D, Fig. 1).
Figure 1. .
Spherical equivalent refractive state (D) and VC depth (mm) over time on treated eyes (solid symbols and lines) and contralateral control eyes (empty symbols, dotted lines). Solid and empty black symbols: those treated and contralateral eyes, respectively, that remained hyperopic after the end of treatment. Solid and empty red symbols: the animals that had myopia on both eyes after treatment. Grey cloud: 95% confidence interval (CI) from age-matched untreated marmosets, calculated as the average ±2 SD. Note that several eyes began the experiment with slightly more myopia than that normally seen in control animals, but the eye size as measured as VC depth fell within normal range.
Figure 2 describes the interocular changes in refraction and vitreous chamber over time, where it can be seen that four weeks into treatment (at T1) five of 10 experimental eyes were relatively more hyperopic than their contralateral control eyes (exp-con mean MSE ± SE +1.79 ± 0.52 mm), and seven were relatively smaller (exp-con mean VC ± SE −0.09 ± 0.02 mm). At T2, six of those seven animals still had smaller eyes (exp-con mean VC ± SE −0.08 ± 0.01 mm, Fig. 2), but now nine were relatively more hyperopic on their treated than contralateral eyes (exp-con mean MSE ± SE +1.76 ± 0.34 mm, Fig. 2). Those nine animals remained relatively more hyperopic in their experimental than control eyes at T3 (exp-con mean MSE ± SE +1.32 ± 0.21 mm, Fig. 2), but two more experimental eyes grew less than their contralateral eyes and now a total of eight animals had smaller experimental than control eyes (exp-con mean VC ± SE −0.06 ± 0.01 mm, Fig. 2). During the last two weeks of treatment, those same eight animals exhibited reduced VC growth in their experimental eyes (exp-con mean VC ± SE −0.06 ± 0.01 mm, Fig. 2), and seven were more hyperopic (exp-con mean MSE ± SE +1.21 ± 0.47 mm, Fig. 2).
Figure 2. .
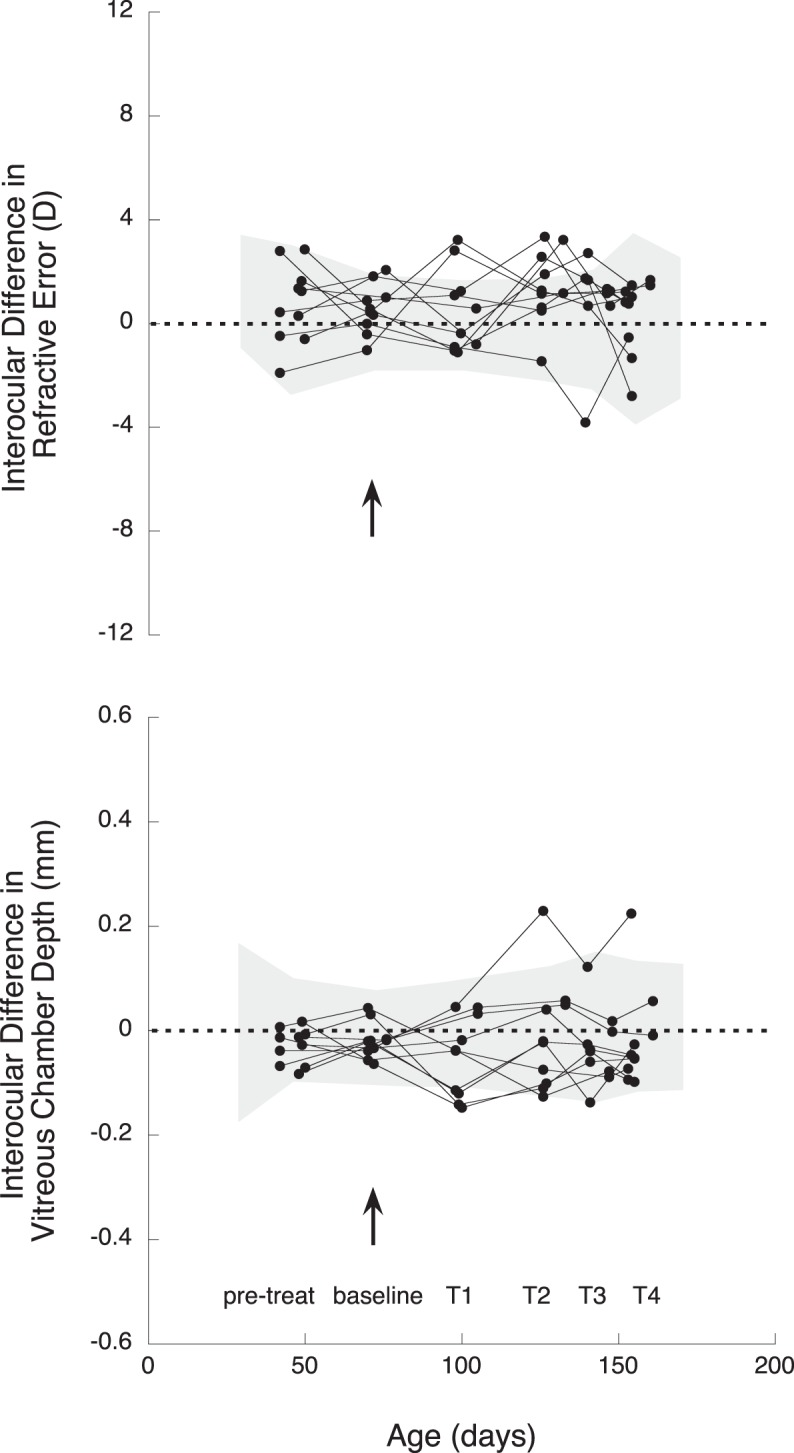
Interocular differences (treated eye-control eye) at pretreatment (pre-treat), baseline (arrow), and during treatment (T1–T4) in refractive error (D) and VC depth (mm) in multizone lens-reared animals. Each line represents one animal, and each symbol indicates the measurement point in time. Grey cloud: 95% CI from age-matched untreated marmosets, calculated as the average ±2 SD.
The refractive state at baseline did not correlate with the refractive state after treatment (R2 = 0.23, P = 0.16) or the growth rates experienced during treatment (R2 = 0.18, P = 0.22).
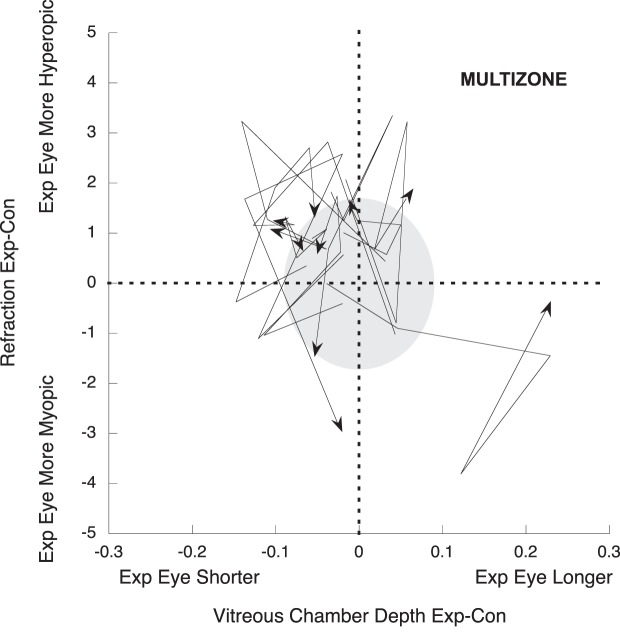
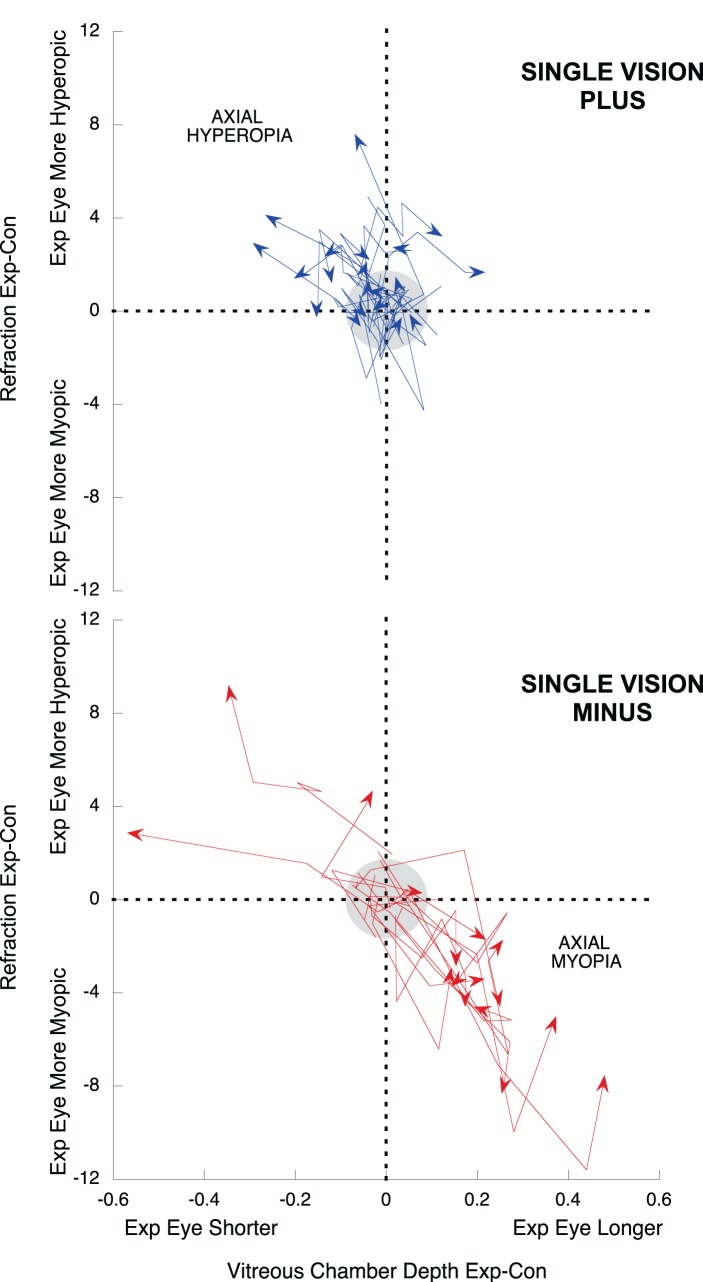
A quadrant plot was used to display visually the changes in interocular refraction and vitreous chamber depth over time in multizone lens-reared animals (Fig. 3), and similar quadrant plots allowed the comparison with negative and positive-single vision lens-reared animals (Fig. 4).
Figure 3. .
Quadrant plot describing the relation between interocular differences (exp-control) in vitreous chamber depth (x axis), and refraction (y axis) in the multizone-lens reared group. The refractive and growth changes of each animal are represented with a line that starts at the baseline measurement and finishes at the last measurement after treatment (arrowhead), plotted over a grey cloud that represents the 95% CI of the age-matched untreated data. Lines outside of the 95% CI indicate significant changes. Data points in the top left quadrant indicate eyes that are smaller and more hyperopic than contralateral control eyes, points in the top right quadrant indicate eyes that are larger but more hyperopic, points in the bottom left quadrant show eyes that are smaller but more myopic than contralateral controls, and points in the bottom right quadrant show eyes that are larger and more myopic.
Figure 4. .
Quadrant plots describing the relation between interocular differences (exp-control) in vitreous chamber depth (x axis), and refraction (y axis) in the positive single-vision reared group (blue arrows), and in the negative single-vision reared group (red arrows). For detailed explanation of the graphs, see Figure 3 caption.
Figure 3 shows how at the end of treatment (represented by an arrowhead), six of 10 multizone lens-treated animals had smaller and relatively more hyperopic experimental than control eyes. These compensatory changes qualitatively resembled the effects of those raised with single vision positive defocus (Fig. 4, exp-con mean MSE ± SE multizone +0.38 ± 0.46 D, positive +1.62 ± 0.44 D, P = 0.10; exp-con mean VC ± SE multizone −0.02 ± 0.03 mm, positive −0.06 ± 0.03 mm, P = 0.35), and were unlike those of single vision negative lens-reared animals, whose treated eyes were bigger and more myopic than the contralateral controls (Fig. 4, exp-con mean MSE ± SE multizone +0.38 ± 0.46 D, negative −2.13 ± 1.10D, P = 0.048; exp-con mean VC ± SE multizone −0.02 ± 0.03 mm, negative +0.12 ± 0.06 mm, P = 0.06).
The overall relation between interocular differences in vitreous chamber depth and refraction over time also can be noticed by the diagonal trend of the data in Figure 3 (R2 = −0.66, P < 0.001). The correlation remained significantly unchanged at each measurement during treatment and compared to baseline (ANOVA repeated measures, P > 0.05). Changes in CC, ACD, LT, RT, or CT did not change significantly during treatment (Table 2), and did not correlate with refractive state or vitreous chamber changes (all P > 0.05).
Table 2. .
Interocular Differences in Biometry and Mean Spherical Equivalent Refraction over Time (Treated Eye-Control Eye, Mean ± SE): Pretreatment, Baseline, T1, T2, T3, and T4
|
|
Pretreatment |
Baseline |
T1 |
T2 |
T3 |
T4 |
| MSE, D | −0.118 ± 0.369 | 0.099 ± 0.357 | 0.935 ± 0.478 | 0.545 ± 0.553 | 0.834 ± 0.401 | 0.576 ± 0.618 |
| VCD, mm | −0.029 ± 0.011 | −0.019 ± 0.011 | −0.049 ± 0.024 | −0.008 ± 0.034 | −0.033 ± 0.022 | −0.017 ± 0.030 |
| CC, mm | −0.005 ± 0.027 | −0.003 ± 0.005 | 0.013 ± 0.012 | 0.017 ± 0.018 | 0.015 ± 0.019 | −0.001 ± 0.007 |
| ACD, mm | −0.011 ± 0.013 | 0.005 ± 0.011 | −0.029 ± 0.012 | −0.016 ± 0.016 | −0.017 ± 0.011 | 0.001 ± 0.008 |
| CT, mm | −0.005 ± 0.027 | −0.003 ± 0.005 | 0.013 ± 0.012 | 0.017 ± 0.018 | 0.015 ± 0.019 | −0.001 ± 0.007 |
| LT, mm | 0.019 ± 0.019 | −0.018 ± 0.017 | 0.005 ± 0.008 | 0.008 ± 0.015 | 0.000 ± 0.009 | −0.022 ± 0.012 |
| RT, mm | −0.010 ± 0.007 | 0.010 ± 0.015 | −0.003 ± 0.005 | −0.003 ± 0.005 | 0.003 ± 0.007 | 0.001 ± 0.004 |
| ChT, mm | 0.010 ± 0.007 | −0.007 ± 0.007 | 0.008 ± 0.005 | 0.000 ± 0.005 | −0.005 ± 0.007 | −0.008 ± 0.005 |
VCD, VC depth; ChT, choroidal thickness.
The temporal characteristics of the effect that multizone lenses had compared to untreated, single vision negative and positive lenses showed no significant differences in growth rates between treatment groups before treatment started (P = 0.36, Fig. 5). However, six weeks into treatment the interocular growth rates of multizone lens-reared animals were significantly lower than those of single vision negative lenses (growth rate mean ± SE multizone −1.0 ± 0.1 μm/day, negative single vision +2.1 ± 0.9 μm/day, P = 0.024, Fig. 5), but similar to untreated (growth rate mean ± SE −0.2 ± 0.4 μm/day, P = 0.33, Fig. 5) and single vision positive lenses (growth rate mean ± SE −2.2 ± 0.7 μm /day, P = 0.37, Fig. 5). Later, between six and eight weeks of treatment, only the rates between single vision positive and negative lens-treated marmosets remained significantly different, with negative single vision lens-reared animals exhibiting higher interocular growth rates than positive, essentially zero at that time (growth rate-negative, lens-treated mean ± SE +2.7 ± 1.1 μm/day, positive lens-treated −9.8439 e−3 ± 0.5 μm/day, P = 0.043, Fig. 5). After eight weeks of treatment all treatment groups showed similar interocular growth rates (P > 0.05, Fig. 5).
Figure 5. .
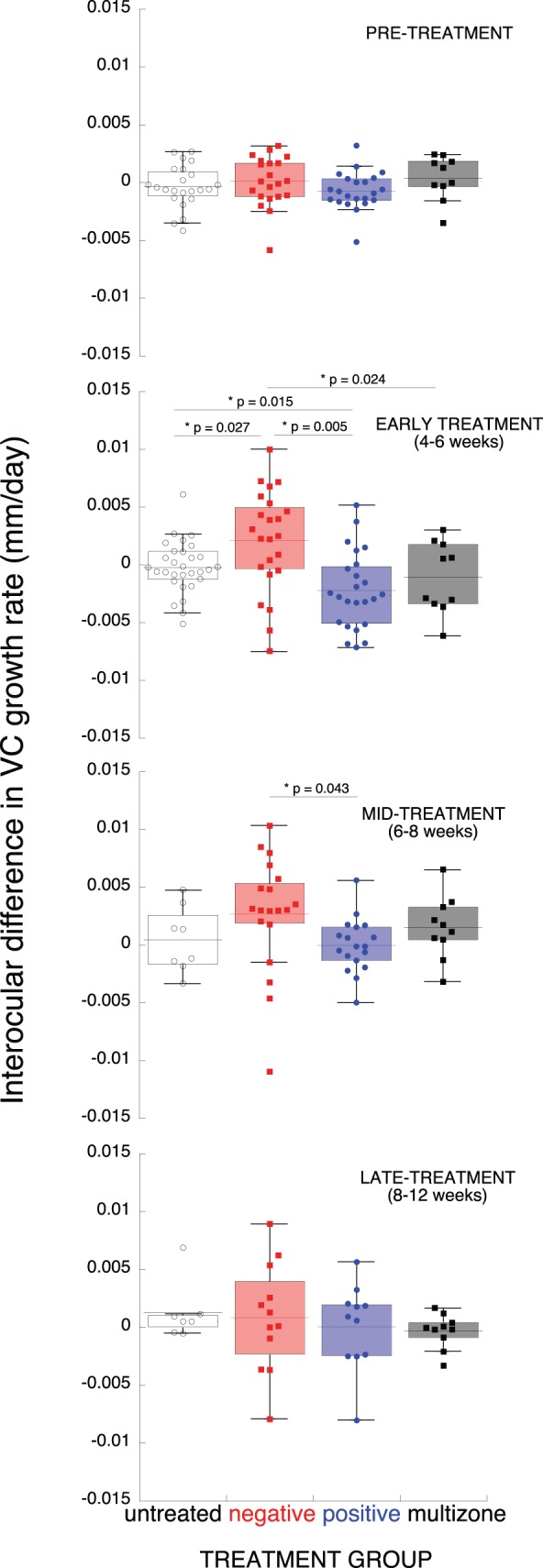
Growth rates in multizone lens-reared marmosets (black), untreated (white), and single vision lens-reared marmosets (positive, blue and negative, red). Before treatment (pretreatment) and at early, mid, and late time intervals during treatment. The data are given as box plots, where the top and bottom end of the box represent the 25th and 75th percentile of the data, and the error bars represent the SD. The underlying dots represent individual data points, and the horizontal line represents the mean.
Discussion
Imposing mixed myopic and hyperopic defocus simultaneously using concentric multizone contact lenses tended to result, overall, in relatively more hyperopic refractions and slower growth rates in treated than fellow eyes. The effects on the vitreous chamber growth rates and refractive development differed from single vision negative lens-reared animals, and were comparable to those of single vision positive lens-reared and untreated animals.
The outcomes of our study suggested that the marmoset, like the chick,21,22 can integrate defocus of competing sign presented simultaneously. The multizone contact lens was designed to provide a 50:50 ratio of hyperopic-to-myopic defocus for an average pupil of 2.8 mm diameter, but the pupils of the marmosets treated in our study were significantly smaller, 2.26 to 2.76 mm, which resulted in greater overall exposure to hyperopic than myopic defocus. Despite the fact that the smaller pupil diameters led to greater percentage of hyperopic defocus, seven of 10 animals had smaller vitreous chambers in their treated than in their contralateral control eyes as early as four weeks into treatment. The effects decreased but remained by the end of treatment. These results suggested that a relatively smaller percentage of myopic defocus imposed simultaneously along a larger percentage of hyperopic defocus leads to relatively smaller and less myopic eyes.
Despite the relatively reduced growth response in eyes with mixed defocus compared to the contralateral control eyes, at the end of treatment six monkeys were myopic in both eyes. Three of these were myopic at baseline, so it could be argued that the final refractive state was related to their baseline refraction. However, a significant correlation was not found between baseline and final refraction. Another possibility is that the subset of treated eyes in fact compensated for the imposed hyperopic defocus in the multizone contact lens, but the average compensatory change in refraction after treatment was less than five diopters, suggesting that the response to negative defocus was reduced by the simultaneously imposed myopic defocus. It also could be possible that the contrast information of the retinal image, affected by the presence of five transition zones and the alternation of six rings of opposite power, behaved as a myopia-inducing stimulus,21,33,34 or the multizone lenses acted like diffusers and affected form vision enough to trigger mild form deprivation myopia.7,13,35 It is important to note that three of the six animals that had myopia in both eyes were less myopic in their treated eye compared to the contralateral control eye at the end of treatment. Because only one of these animals was myopic before treatment, we concluded that the interocular difference during treatment was the result of the positive defocus in the multizone lens; however, we cannot rule out the possibility that the experimental lens had a contralateral effect on the control eye because of yoked accommodation, which has been described in the past,7 and requires further investigation.
The myopic shift observed in four of the 10 contralateral eyes (−3.79 ± 0.71 D) also might have been due to a residual refractive effect related to the fit and centration of the plano contact lens. However, the overall refractive change induced by plano contact lenses was only significant at 40 degrees on the temporal retina (−1.22 ± 0.33 D), and does not entirely explain the overall myopic shift.
The multizone contact lens effects were less strong and also more transient than those treated with single vision lenses (Figs. 4, 5), and they should be interpreted with caution due to the variability between animals and the contralateral effects observed (Fig. 1). The differences in the temporal characteristics between responses to mixed and single vision defocus might be linked to interactions between the temporal response properties to hyperopic and myopic defocus, known to behave in a nonlinear manner.33,36
The accommodative response through the multizone lens could not be recorded in our study, but accommodation through positive and negative single vision lenses has been measured in marmosets before,7 and the results showed that most of the negative-lens-treated animals did not accommodate through the lens and so experienced effective hyperopic defocus during treatment, while a small percentage of animals sporadically accommodated through the single vision negative lens and cleared the defocus. Therefore, we speculate that the marmosets in our study most likely did not accommodate at near and used instead the most myopic image of the treated eye (similar to monovision), leaving the fellow eye exposed to more hyperopic defocus because of yoked accommodation. Supporting this hypothesis, accommodation under imposed anisometropia in humans appears to behave so that the eye with less hyperopia dominates and drives the response, leaving the eye with relatively more imposed hyperopic defocus effectively hyperopic (Benavente-Perez A, et al. IOVS 2010;51: ARVO E-Abstract 3932). This mechanism might explain the effects we observed in the marmosets in our study, where not only the multizone lens-treated eyes, but also the contralateral eyes wearing plano contact lenses increased their axial growth rates and became myopic. There is, however, evidence from other human studies suggesting that accommodation can occur at near for the more hyperopic image of a bifocal contact lens.27,37 Therefore, we cannot rule out that the marmoset eyes treated with the multizone contact lenses might do this on occasion. If this were the case, the contralateral eyes also would have accommodated because of the consensual nature of accommodation, and would have experienced myopic defocus at least some of the time.
The similarities between our study and a preliminary multizone soft contact lens trial in humans27 suggested that multizone designs with a positive addition may be effective in slowing myopia progression in humans. Similar to the response given by our animal model, and despite the variability in refraction and axial length changes, the overall changes in refractive state and axial growth rate were significantly slower in the eyes wearing the multizone lens (referred to as dual focus, DF) than in those eyes wearing single vision (SV) lenses (DF prescription changes −0.44 ± 0.33 D, SV −0.69 ± 0.38, P < 0.001. DF axial length changes 0.11 ± 0.09 mm, SV 0.22 ± 0.10 mm, P < 0.001). The agreement in outcomes between the results obtained in humans and marmosets suggested that when myopic defocus is presented simultaneously with a clear image (humans) or hyperopic defocus (marmosets), the signal is sufficient to reduce eye growth, as reported previously in chicks.21,22 The treatment duration in humans was 20 months and it appeared to slow down the progression of myopia in 70% of the children, which is a greater percentage than the outcomes obtained in past optical manipulation trials.38–41 The myopic defocus induced by the DF at distance and near design is thought to be responsible for the efficacy of this treatment; however, the therapeutic effects of such bifocal designs must be investigated over longer periods of time. If imposing positive defocus peripherally is effective, adding plus to the periphery is a promising optical manipulation, as not only it does not seem to affect VA or contrast sensitivity,27 but the patient also might tolerate it better than undercorrection, progressive, or bifocal lenses.
In conclusion, imposing positive and negative defocus simultaneously on the marmoset eye results in relatively slower growth rates and more hyperopia, despite treated eyes being exposed to greater amounts of negative defocus. The response to mixed defocus is less strong, and more transient than that to full-field defocus, but it indicates that it is possible to control eye growth and refractive error development in the primate model by manipulating the visual environment using optical treatments of combined refractive nature.
Acknowledgments
Josh Wallman provided comments and suggestions, and Nancy Coletta provided earlier discussions on the lens designs.
Footnotes
Supported by the National Institutes of Health (NIH R01 EY11228).
Disclosure: A. Benavente-Perez, None; A. Nour, None; D. Troilo, None
References
- 1.Troilo D, Judge S. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vision Res. 1993;33:1311–1324 [DOI] [PubMed] [Google Scholar]
- 2.Graham B, Judge SJ. Normal development of refractive state and ocular component dimensions in the marmoset (Callithrix jacchus). Vision Res. 1999;39:177–187 [DOI] [PubMed] [Google Scholar]
- 3.Troilo D, Nickla DL. Visual regulation of eye growth and refractive state in a new world primate. In: Thorn F, Troilo D, Gwiazda J.eds Myopia 2000: Proceedings of the VIII International Conference on Myopia. Boston, MA: Myopia 2000, Inc.; 2000:170–174 [Google Scholar]
- 4.Troilo D, Nickla DL, Wildsoet CW. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41:1249–1258 [PubMed] [Google Scholar]
- 5.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–273 [DOI] [PubMed] [Google Scholar]
- 6.Troilo D, Quinn N, Baker K. Accommodation and induced myopia in marmosets. Vision Res. 2007;47:1228–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troilo D, Totonelly K, Harb EN. Imposed anisometropia, accommodation, and regulation of refractive state. Optom Vis Sci. 2009;86:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid KL, Wildsoet CF. Effects on compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036 [DOI] [PubMed] [Google Scholar]
- 9.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek L. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77 [DOI] [PubMed] [Google Scholar]
- 10.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987;6:993–999 [DOI] [PubMed] [Google Scholar]
- 11.Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668 [DOI] [PubMed] [Google Scholar]
- 12.Smith EL 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith EL 3rd, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50:5057–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaeffel F, Troilo D, Wallman J, Howland HC. Developing eyes that lack accommodation grow to compensate for imposed defocus. Visual Neurosci. 1990;4:177–183 [DOI] [PubMed] [Google Scholar]
- 15.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250 [DOI] [PubMed] [Google Scholar]
- 16.Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003;27:371–385 [DOI] [PubMed] [Google Scholar]
- 17.Smith EL 3rd, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43:291–299 [PubMed] [Google Scholar]
- 18.Kee CS, Hung LF, Qiao-Grider Y, et al. Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48:957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Wildsoet C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci. 2011;52:1078–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLean RC, Wallman J. Severe astigmatic blur does not interfere with spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:449–457 [DOI] [PubMed] [Google Scholar]
- 21.Diether S, Wildsoet CF. Stimulus requirements for the decoding of myopic and hyperopic defocus under single and competing defocus conditions in the chicken. Invest Ophthalmol Vis Sci. 2005;46:2242–2252 [DOI] [PubMed] [Google Scholar]
- 22.Tse DY, Lam CS, Guggenheim JA, et al. Simultaneous defocus integration during refractive development. Invest Ophthalmol Vis Sci. 2007;48:5352–5359 [DOI] [PubMed] [Google Scholar]
- 23.Siegwart JJ Jr, Norton T. Binocular lens treatment in tree shrews: effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8:1–12 [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Park T, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46:2238–2241 [DOI] [PubMed] [Google Scholar]
- 26.Zhu X, Wallman J. Temporal properties of compensation for positive and negative spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011;118:1152–1156 [DOI] [PubMed] [Google Scholar]
- 28.Mutti DO, Hayes JR, Michell GL, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48:2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mutti DO, Mitchell GL, Jones LA, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–3080 [DOI] [PubMed] [Google Scholar]
- 30.Tian Y, Tarrant J, Wildsoet C. Optical and biometric characteristics of anisomyopia in human adults. Ophthal Physiol Opt. 2011;31:540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus). Invest Ophthalmol Vis Sci. 2000;41:2043–2049 [PubMed] [Google Scholar]
- 32.Troilo D, Nickla D. The response to form deprivation by occluders differs from that by lid suture in marmosets. Invest Ophthalmol Vis Sci (ARVO Suppl). 2000;41:S134 [Google Scholar]
- 33.Tran N, Chiu S, Tian Y, Wildsoet CF. The significance of retinal image contrast and spatial frequency composition for eye growth modulation in young chicks. Vision Res. 2008;48:1655–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diedrich E, Schaeffel F. Spatial resolution, contrast sensitivity and sensitivity to defocus of chicken retinal ganglion cells in vitro. Visual Neurosci. 2009;26:467–476 [DOI] [PubMed] [Google Scholar]
- 35.Smith EL 3rd, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468 [DOI] [PubMed] [Google Scholar]
- 37.Tarrant J, Severson H, Wildsoet CF. Accommodation in emmetropic and myopic young adults wearing bifocal soft contact lenses. Ophthalmic Physiol Opt. 2008;28:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung K, Mohidin N, O'Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42:2555–2559 [DOI] [PubMed] [Google Scholar]
- 39.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500 [DOI] [PubMed] [Google Scholar]
- 40.Yang Z, Lan W, Ge J, et al. The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthal Physiol Opt. 2009;29:41–48 [DOI] [PubMed] [Google Scholar]
- 41.Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77:395–401 [DOI] [PubMed] [Google Scholar]