Abstract
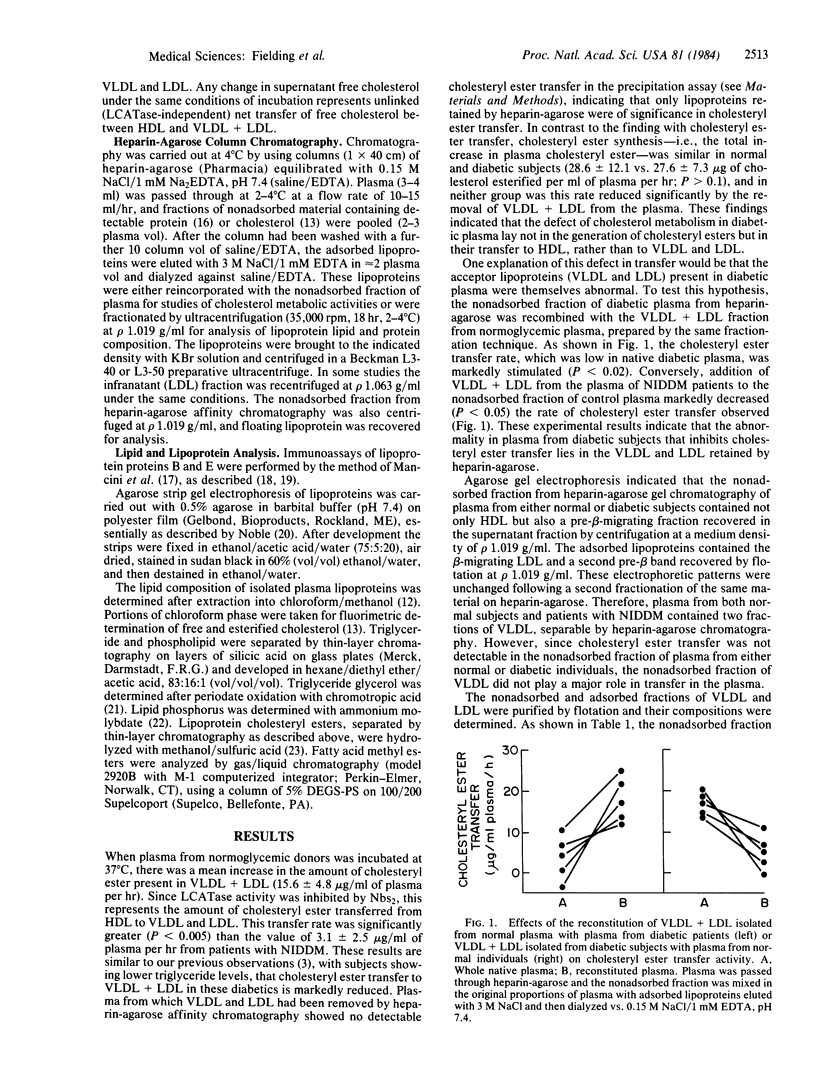
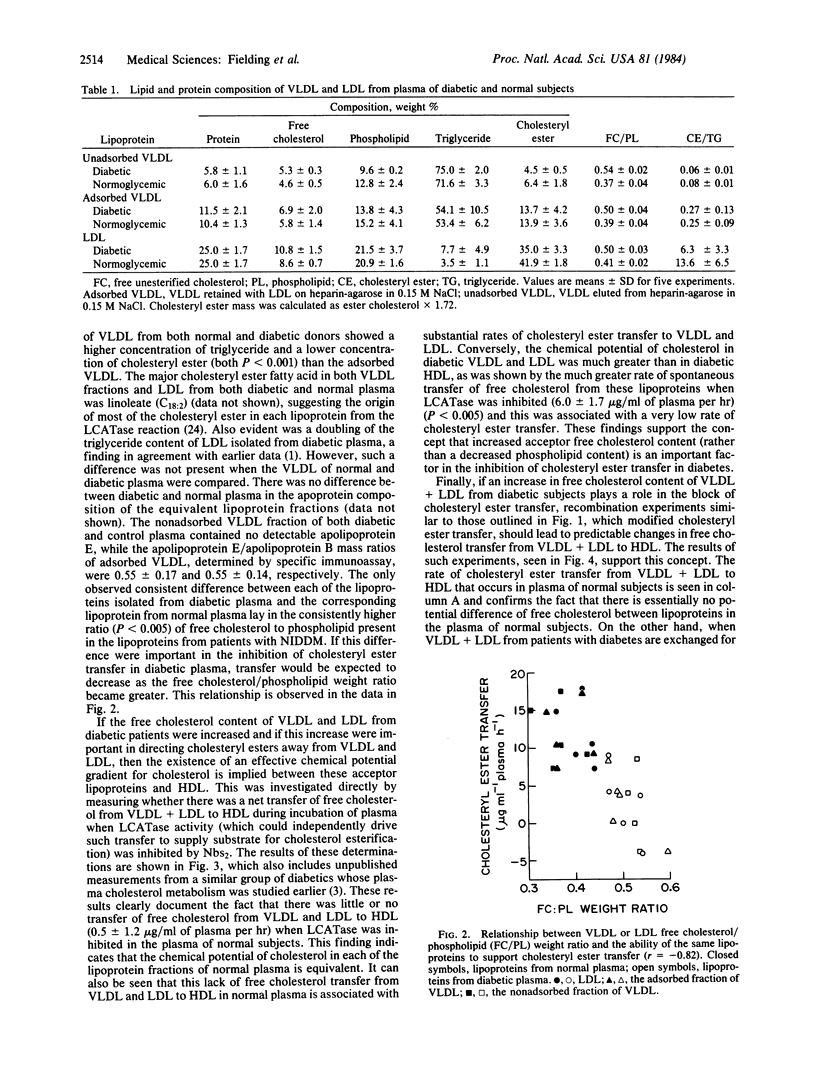
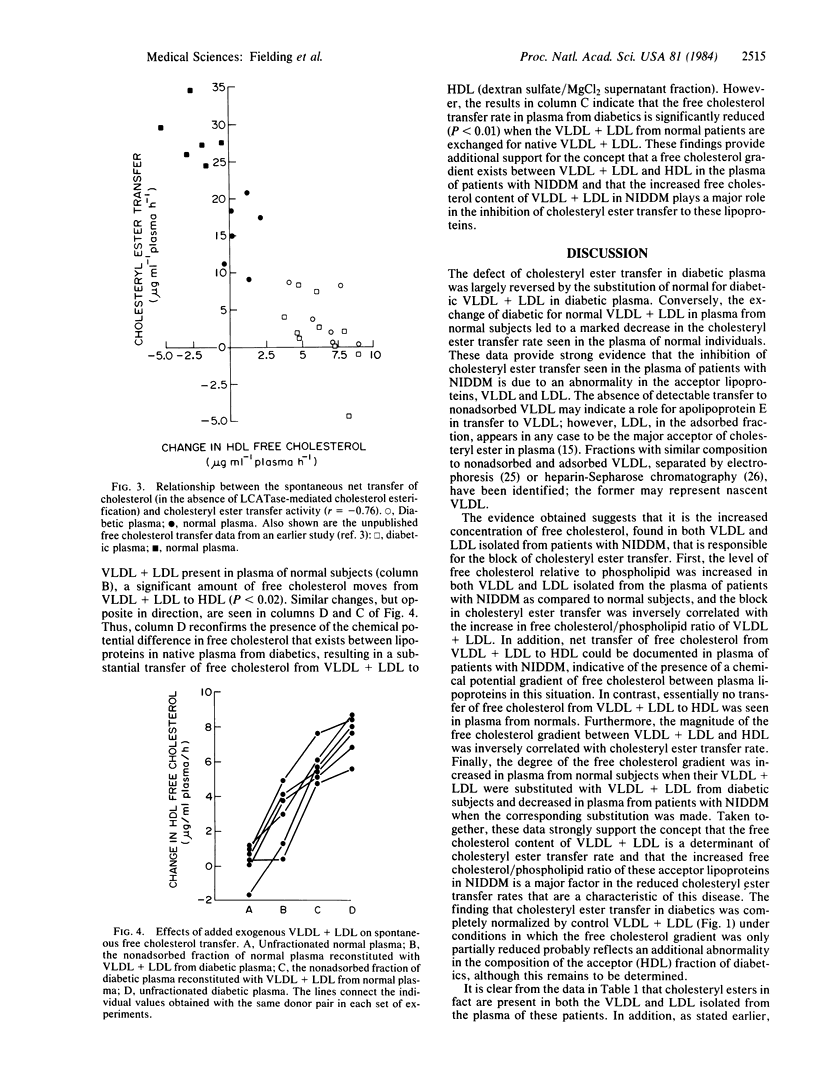
Recombination of low and very low density lipoproteins (VLDL and LDL) from normal subjects with plasma from patients with non-insulin-dependent diabetes mellitus significantly increased the reduced rate of transfer of cholesteryl ester to these lipoproteins, which is characteristic of diabetic plasma, whereas diabetic VLDL and LDL reduced cholesteryl ester transfer rates in normal plasma. VLDL and LDL from diabetic plasma had an increased ratio of free cholesterol to phospholipid compared to normal, and unlike normal VLDL and LDL spontaneously lost free cholesterol to high density lipoprotein. These data suggest that the block to cholesteryl ester transfer to these lipoproteins in non-insulin-dependent diabetes is mediated by their increased free cholesterol content and may be related to the increased risk of these patients for developing atherosclerosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- CARLSON L. A. DETERMINATION OF SERUM TRIGLYCERIDES. J Atheroscler Res. 1963 Jul-Aug;3:334–336. doi: 10.1016/s0368-1319(63)80012-5. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Regulation of human plasma lecithin:cholesterol acyltransferase activity by lipoprotein acceptor cholesteryl ester content. J Biol Chem. 1981 Mar 10;256(5):2102–2104. [PubMed] [Google Scholar]
- Fielding C. J., Frohlich J., Moser K., Fielding P. E. Promotion of sterol efflux and net transport by apolipoprotein E in lecithin:cholesterol acyltransferase deficiency. Metabolism. 1982 Oct;31(10):1023–1028. doi: 10.1016/0026-0495(82)90146-9. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Reaven G. M., Fielding P. E. Human noninsulin-dependent diabetes: identification of a defect in plasma cholesterol transport normalized in vivo by insulin and in vitro by selective immunoadsorption of apolipoprotein E. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6365–6369. doi: 10.1073/pnas.79.20.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J. A cholesteryl ester transfer complex in human plasma. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3327–3330. doi: 10.1073/pnas.77.6.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J., Havel R. J., Kane J. P., Tun P. Cholesterol net transport, esterification, and transfer in human hyperlipidemic plasma. J Clin Invest. 1983 Mar;71(3):449–460. doi: 10.1172/JCI110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider J. G., Boyett R. L. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978 May;19(4):514–518. [PubMed] [Google Scholar]
- Howard B. V., Savage P. J., Bennion L. J., Bennett P. H. Lipoprotein composition in diabetes mellitus. Atherosclerosis. 1978 Jun;30(2):153–162. doi: 10.1016/0021-9150(78)90058-8. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Castelli W. P., Gordon T., McNamara P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971 Jan;74(1):1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972 Sep;223(3):715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Pagnan A., Havel R. J., Kane J. P., Kotite L. Characterization of human very low density lipoproteins containing two electrophoretic populations: double pre-beta lipoproteinemia and primary dysbetalipoproteinemia. J Lipid Res. 1977 Sep;18(5):613–622. [PubMed] [Google Scholar]
- Sata T., Havel R. J., Jones A. L. Characterization of subfractions of triglyceride-rich lipoproteins separated by gel chromatography from blood plasma of normolipemic and hyperlipemic humans. J Lipid Res. 1972 Nov;13(6):757–768. [PubMed] [Google Scholar]
- Schonfeld G., Birge C., Miller J. P., Kessler G., Santiago J. Apolipoprotein B levels and altered lipoprotein composition in diabetes. Diabetes. 1974 Oct;23(10):827–834. doi: 10.2337/diab.23.10.827. [DOI] [PubMed] [Google Scholar]
- Sgoutas D. S. Fatty acid specificity of plasma phosphatidylcholine: cholesterol acyltransferase. Biochemistry. 1972 Jan 18;11(2):293–296. doi: 10.1021/bi00752a022. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Bennett J. S., Colman R. W., Cooper R. A. Abnormalities of cholesterol-phospholipid composition in platelets and low-density lipoproteins of human hyperbetalipoproteinemia. J Lab Clin Med. 1977 Feb;89(2):341–353. [PubMed] [Google Scholar]
- Shelburne F. A., Quarfordt S. H. The interaction of heparin with an apoprotein of human very low density lipoprotein. J Clin Invest. 1977 Oct;60(4):944–950. doi: 10.1172/JCI108849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke K. T., Norum K. R. Determination of lecithin: cholesterol acyltransfer in human blood plasma. Scand J Clin Lab Invest. 1971 Feb;27(1):21–27. doi: 10.3109/00365517109080184. [DOI] [PubMed] [Google Scholar]
- Vakakis N., Redgrave T. G., Small D. M., Castelli W. P. Cholesterol content of red blood cells and low-density lipoproteins in hypertriglyceridemia. Biochim Biophys Acta. 1983 May 16;751(3):280–285. doi: 10.1016/0005-2760(83)90285-0. [DOI] [PubMed] [Google Scholar]
- West K. M., Ahuja M. M., Bennett P. H., Czyzyk A., De Acosta O. M., Fuller J. H., Grab B., Grabauskas V., Jarrett R. J., Kosaka K. The role of circulating glucose and triglyceride concentrations and their interactions with other "risk factors" as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care. 1983 Jul-Aug;6(4):361–369. doi: 10.2337/diacare.6.4.361. [DOI] [PubMed] [Google Scholar]



