Abstract
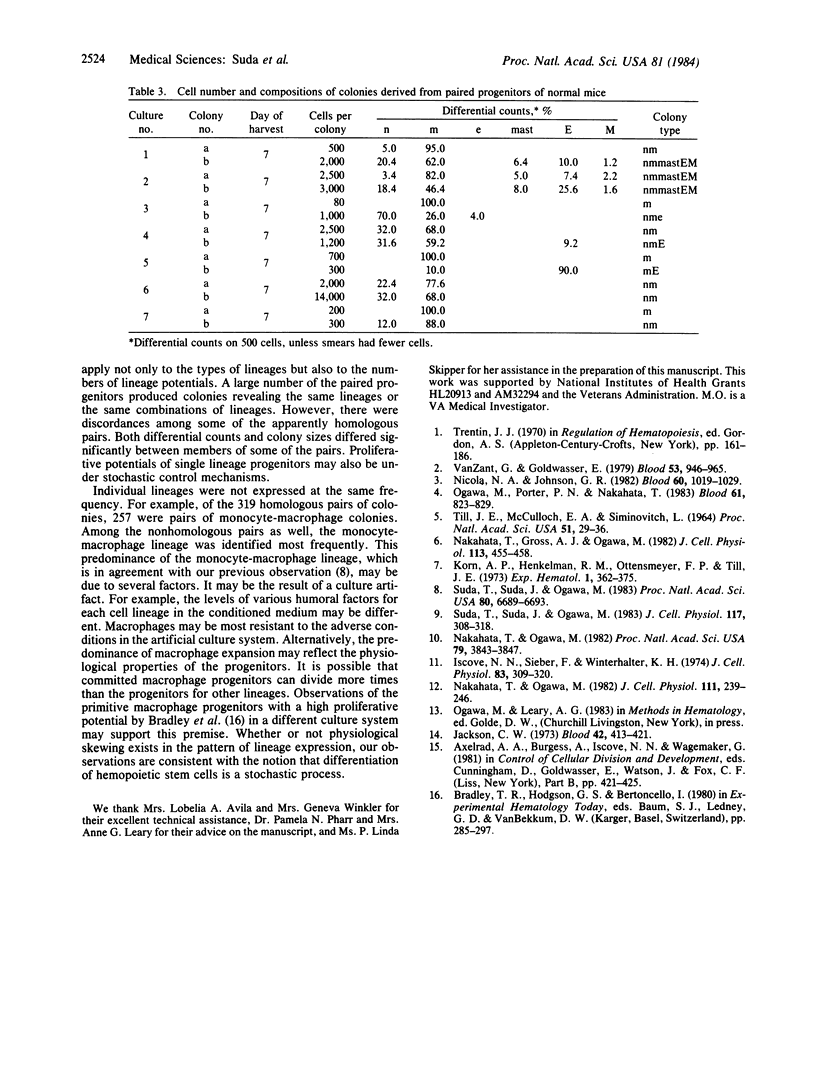
We analyzed the differentiation of murine hemopoietic colonies derived from paired progenitors in culture. Single progenitors were isolated by use of a micromanipulation technique from blast cell colonies cultured from the spleens of 5-fluorouracil-treated mice. Eighteen to 24 hr later, the paired progenitors were separated with a micromanipulator and cultured in methylcellulose medium containing erythropoietin and pokeweed-mitogen spleen cell conditioned medium. Six to nine days later, the two colonies derived from the paired progenitors were individually picked and differential counts were performed by using May-Grunwald-Giemsa stain. The abbreviations used here are n, neutrophil; m, macrophage; e, eosinophil; mast, mast cell; M, megakaryocyte; E, erythrocyte. Of a total of 387 pairs that could be evaluated, 68 were pairs of colonies consisting of dissimilar combinations of cell lineages such as m-nmmastEM, M-nmmastEM, nm-nmmastEM, nmmastM-nmmastEM, M-nmmastM, nmmast-nmmastM, nm-nmmastE, M-nmM, n-nmM, mM-nmM, m-nmmast, nm-nme, me-nm, mM-nm, n-ne, m-mmast, m-mM, M-nm, M-mM, E-nm, m-nm, M-m, etc. Thirty-nine were homologous pairs revealing identical lineage combinations such as nmmastEM, nmmastM, nmmast, mmastEM, nmEM, nme, nmM, mM, and nm lineages. However, in members of some of these pairs, the proportions of the individual cell lineages were significantly different. The remainder were pairs of single lineage colonies. Paired progenitors obtained from the stem cell colonies of normal mice also revealed homologous and nonhomologous expression of the cell lineages. Comparison of lineage expression in colonies derived from single progenitors with the sum of lineages expressed in pairs of colonies derived from single progenitors indicated that the diversity was not due to injury inflicted by micromanipulation. These observations provide experimental data in support of stochastic mechanisms of stem cell differentiation.
Full text
PDF
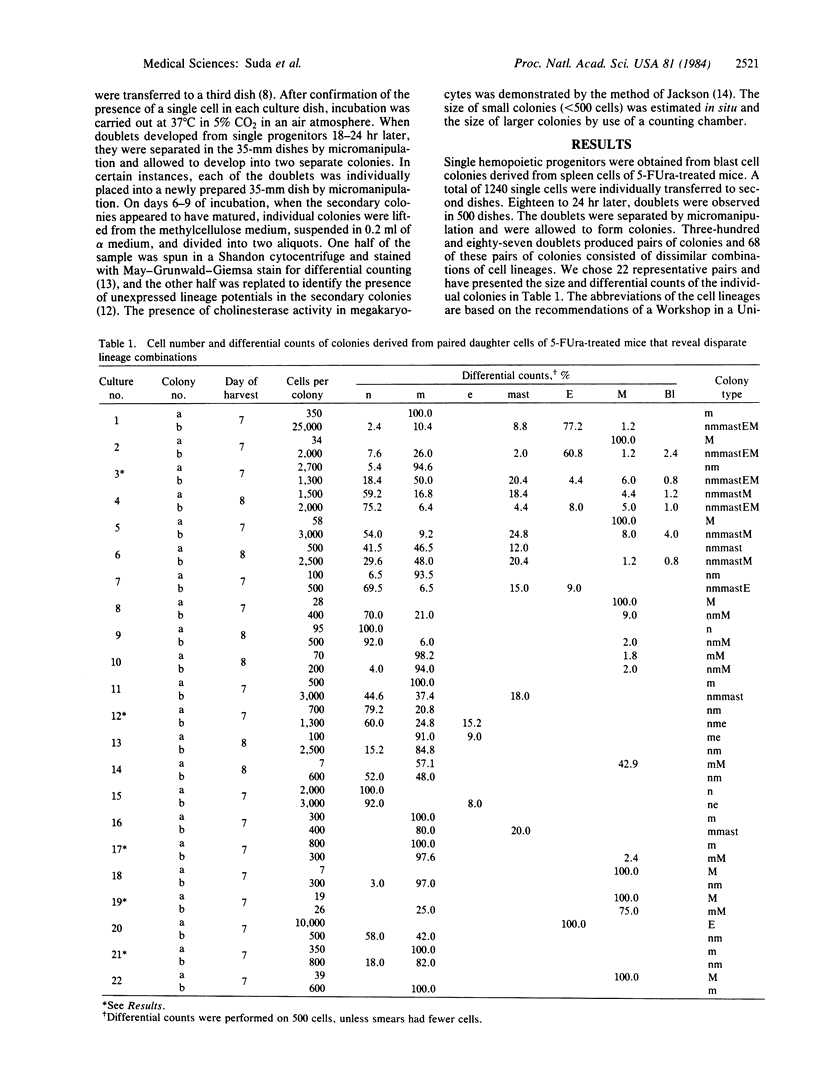
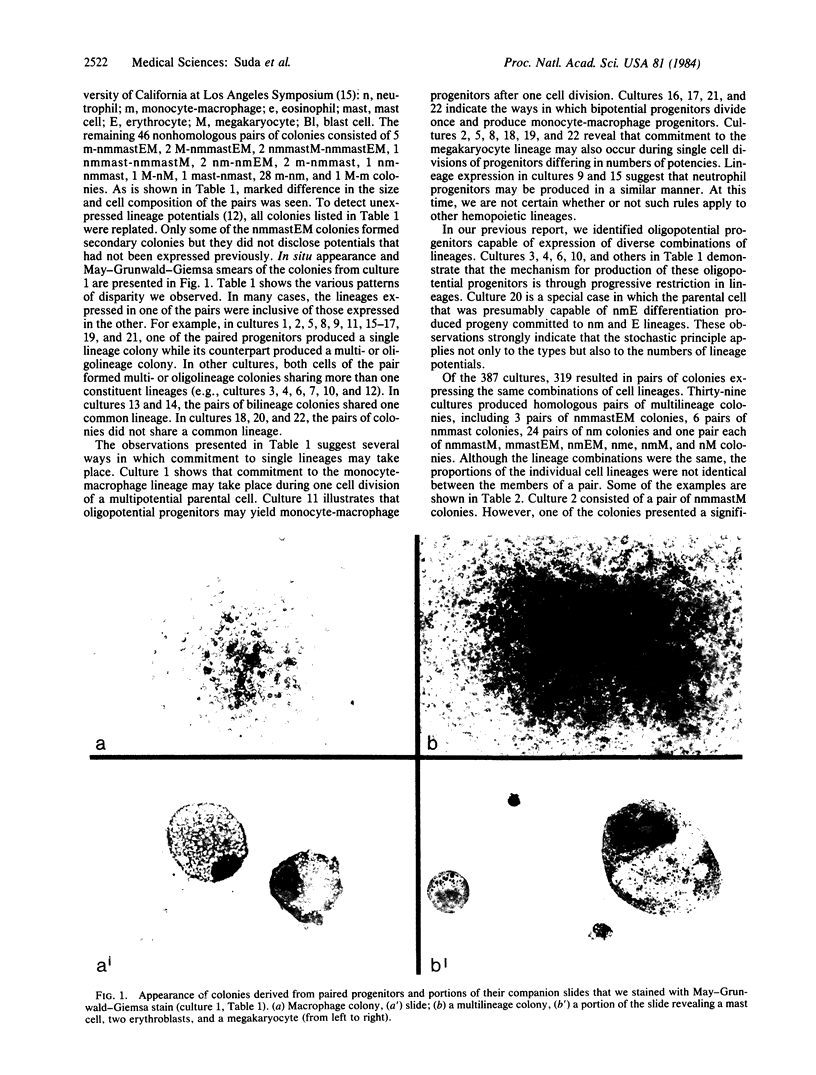
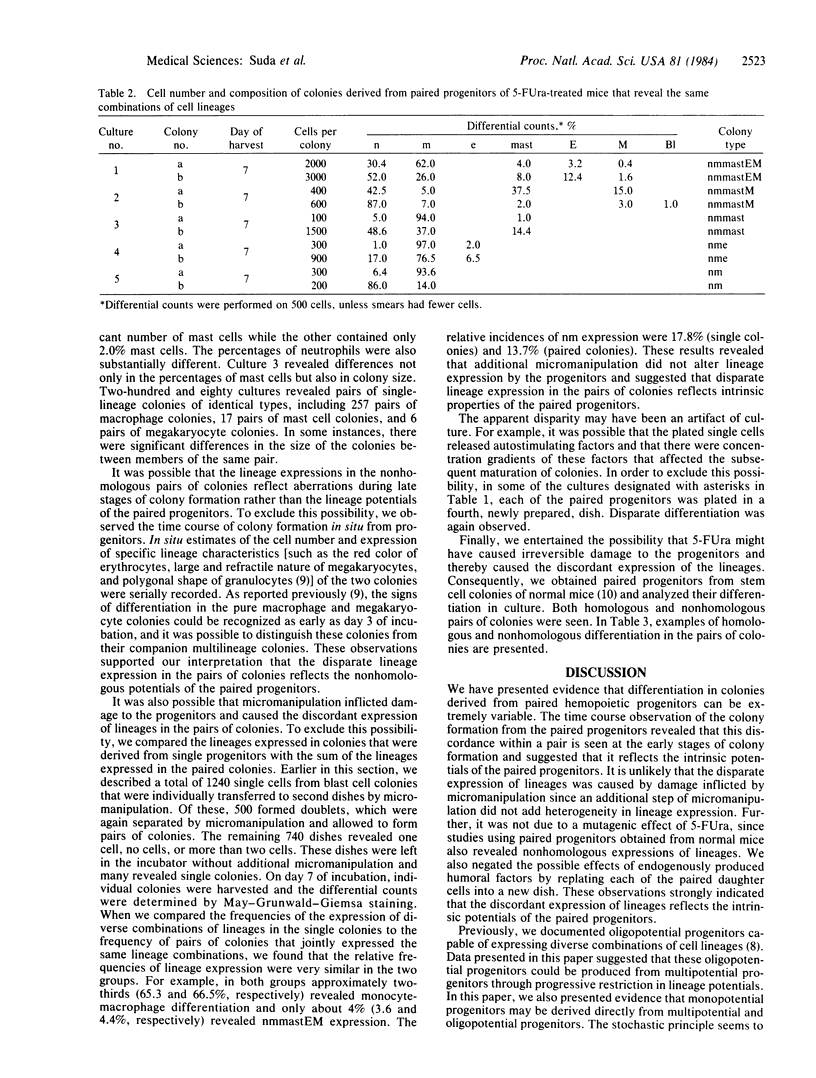
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Jackson C. W. Cholinesterase as a possible marker for early cells of the megakaryocytic series. Blood. 1973 Sep;42(3):413–421. [PubMed] [Google Scholar]
- Korn A. P., Henkelman R. M., Ottensmeyer F. P., Till J. E. Investigations of a stochastic model of haemopoiesis. Exp Hematol. 1973;1(6):362–375. [PubMed] [Google Scholar]
- Nakahata T., Gross A. J., Ogawa M. A stochastic model of self-renewal and commitment to differentiation of the primitive hemopoietic stem cells in culture. J Cell Physiol. 1982 Dec;113(3):455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Clonal origin of murine hemopoietic colonies with apparent restriction to granuclocyte-macrophage-megakaryocyte (GMM) differentiation. J Cell Physiol. 1982 Jun;111(3):239–246. doi: 10.1002/jcp.1041110304. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Johnson G. R. The production of committed hemopoietic colony-forming cells from multipotential precursor cells in vitro. Blood. 1982 Oct;60(4):1019–1029. [PubMed] [Google Scholar]
- Ogawa M., Porter P. N., Nakahata T. Renewal and commitment to differentiation of hemopoietic stem cells (an interpretive review). Blood. 1983 May;61(5):823–829. [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6689–6693. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zant G., Goldwasser E. Competition between erythropoietin and colony-stimulating factor for target cells in mouse marrow. Blood. 1979 May;53(5):946–965. [PubMed] [Google Scholar]