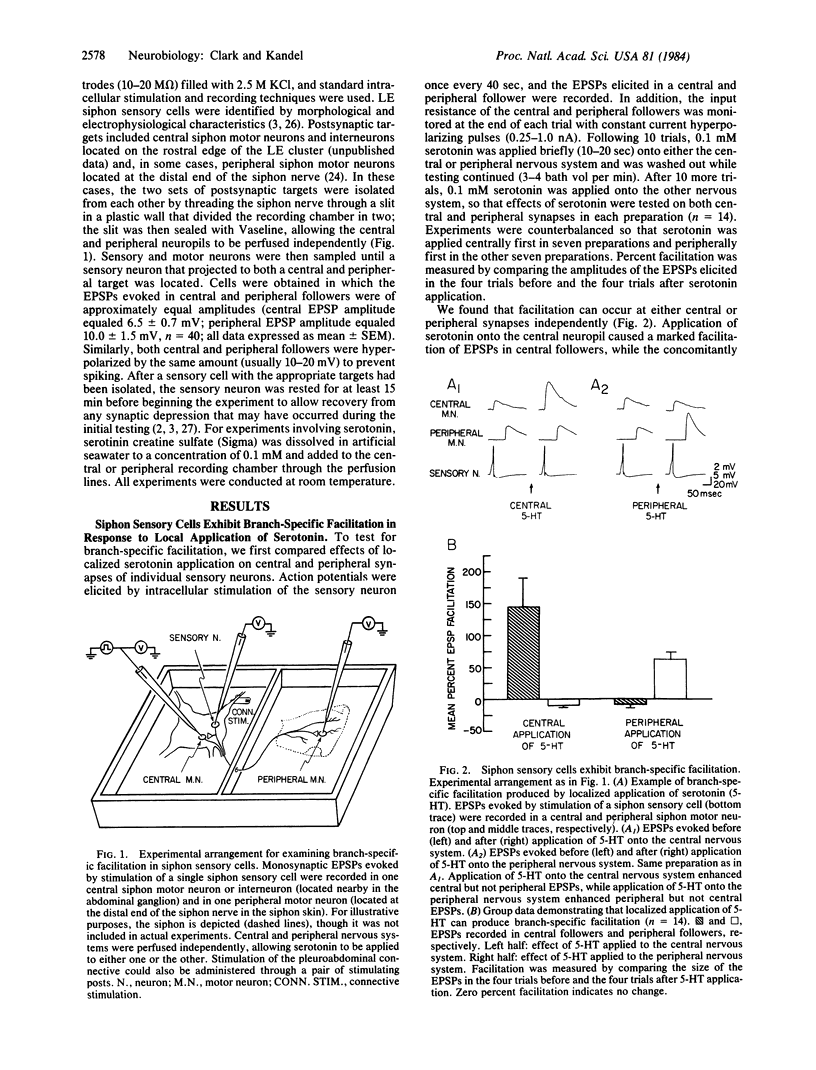
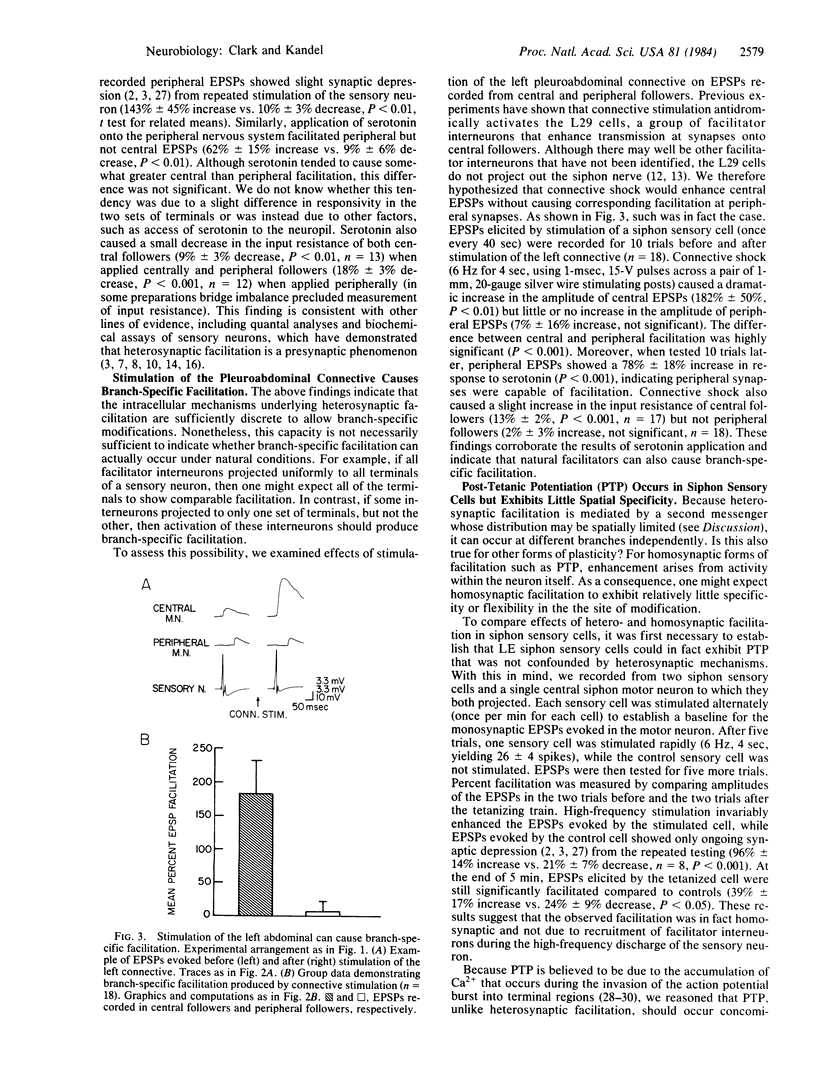
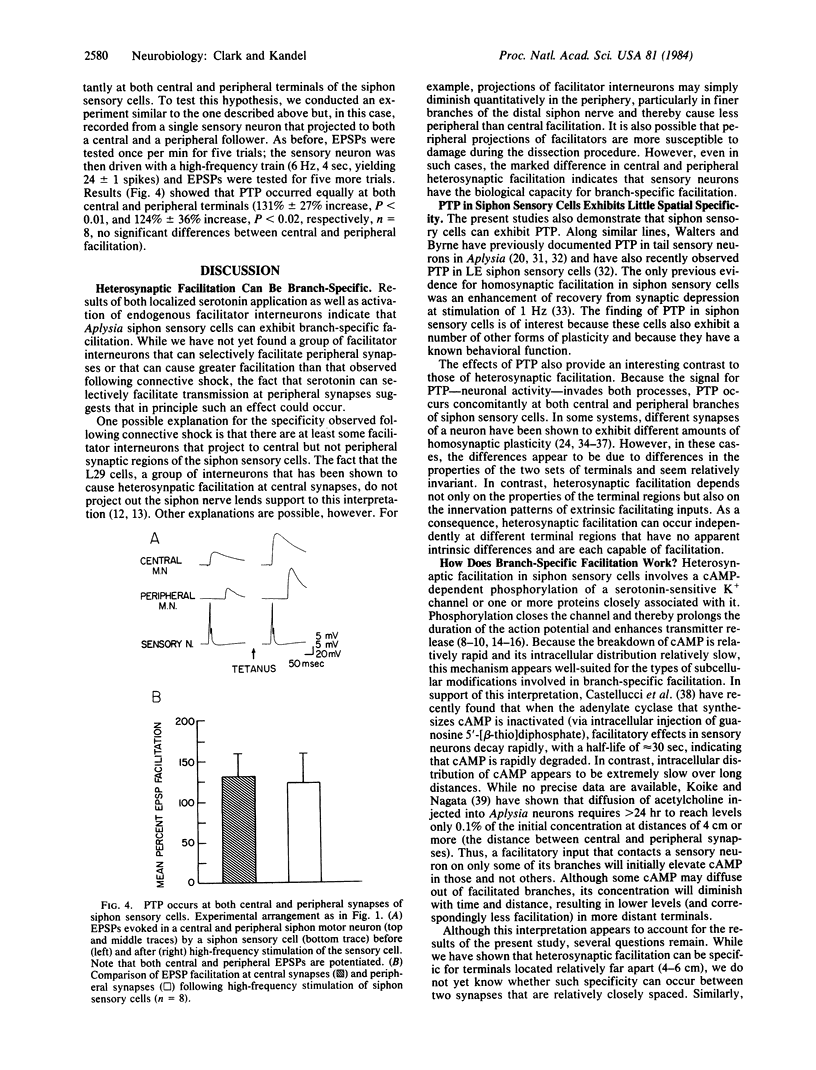
Abstract
Aplysia siphon sensory cells exhibit heterosynaptic facilitation of transmitter release during both sensitization and classical conditioning of the siphon withdrawal response. In the present study, we asked whether facilitation must invariably enhance transmission at all terminals of a neuron or whether facilitation can instead occur at one set of terminals without also occurring at other terminals of the same cell. To examine this question, we compared effects of local application of serotonin and of connective stimulation on transmission at central and peripheral branches of single sensory cells. We found that heterosynaptic facilitation can be branch-specific and can occur at either central or peripheral synapses independently. We also found that siphon sensory cells exhibit homosynaptic post-tetanic potentiation, allowing us to compare effects of hetero- and homosynaptic facilitation in the same cells. By contrast to heterosynaptic facilitation, homosynaptic facilitation occurs concomitantly at both central and peripheral synapses of siphon sensory cells. Thus, while both heterosynaptic and homosynaptic facilitation involve increases in transmitter release from sensory neuron terminals, heterosynaptic facilitation provides a greater specificity and flexibility in the modification of synaptic connections.
Keywords: presynaptic facilitation, post-tetanic potentiation, neuronal plasticity, sensitization, classical conditioning
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood H. L. Variation in physiological properties of crustacean motor synapses. Nature. 1967 Jul 1;215(5096):57–58. doi: 10.1038/215057a0. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Castellucci V. F., Koester J., Kandel E. R. Cellular studies of peripheral neurons in siphon skin of Aplysia californica. J Neurophysiol. 1979 Mar;42(2):530–557. doi: 10.1152/jn.1979.42.2.530. [DOI] [PubMed] [Google Scholar]
- Bernier L., Castellucci V. F., Kandel E. R., Schwartz J. H. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3':5'-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982 Dec;2(12):1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner G. D. Differentiation of nerve terminals in the crayfish opener muscle and its functional significance. J Gen Physiol. 1968 Jun;51(6):731–758. doi: 10.1085/jgp.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Byers D., Davis R. L., Kiger J. A., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981 Jan 1;289(5793):79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- Byrne J. H. Analysis of synaptic depression contributing to habituation of gill-withdrawal reflex in Aplysia californica. J Neurophysiol. 1982 Aug;48(2):431–438. doi: 10.1152/jn.1982.48.2.431. [DOI] [PubMed] [Google Scholar]
- Byrne J., Castellucci V., Kandel E. R. Receptive fields and response properties of mechanoreceptor neurons innervating siphon skin and mantle shelf in Aplysia. J Neurophysiol. 1974 Sep;37(5):1041–1064. doi: 10.1152/jn.1974.37.5.1041. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Hawkins R. D., Kandel E. R. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983 Jan 28;219(4583):397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Walters E. T., Kandel E. R. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981 Dec;1(12):1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Nairn A., Greengard P., Schwartz J. H., Kandel E. R. Inhibitor of adenosine 3':5'-monophosphate-dependent protein kinase blocks presynaptic facilitation in Aplysia. J Neurosci. 1982 Dec;2(12):1673–1681. doi: 10.1523/JNEUROSCI.02-12-01673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Pinsker H., Kupfermann I., Kandel E. R. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Jan Y. N., Byers D., Quinn W. G., Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976 May;73(5):1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E. Matching of facilitation at the neuromuscular junction of the lobster: a possible case for influence of muscle on nerve. J Physiol. 1973 Sep;233(3):635–658. doi: 10.1113/jphysiol.1973.sp010327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORMEZANO I., SCHNEIDERMAN N., DEAUX E., FUENTES I. Nictitating membrane: classical conditioning and extinction in the albino rabbit. Science. 1962 Oct 5;138(3536):33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Castellucci V. F., Kandel E. R. Interneurons involved in mediation and modulation of gill-withdrawal reflex in Aplysia. I. Identification and characterization. J Neurophysiol. 1981 Feb;45(2):304–314. doi: 10.1152/jn.1981.45.2.304. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Castellucci V. F., Kandel E. R. Interneurons involved in mediation and modulation of gill-withdrawal reflex in Aplysia. II. Identified neurons produce heterosynaptic facilitation contributing to behavioral sensitization. J Neurophysiol. 1981 Feb;45(2):315–328. doi: 10.1152/jn.1981.45.2.315. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Nagata Y. Intra-axonal diffusion of [3H]acetylcholine and [3H]gamma-aminobutyric acid in a neurone of Aplysia. J Physiol. 1979 Oct;295:397–417. doi: 10.1113/jphysiol.1979.sp012976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz R., Shapiro E., Kandel E. R. Post-tetanic potentiation at an identified synapse in Aplysia is correlated with a Ca2+-activated K+ current in the presynaptic neuron: evidence for Ca2+ accumulation. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5430–5434. doi: 10.1073/pnas.79.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I., Castellucci V., Pinsker H., Kandel E. Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1743–1745. doi: 10.1126/science.167.3926.1743. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K. J., Nicholls J. G. Different properties of synapses between a single sensory neurone and two different motor cells in the leech C.N.S. J Physiol. 1974 Apr;238(2):357–369. doi: 10.1113/jphysiol.1974.sp010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker H., Kupfermann I., Castellucci V., Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983 Jan 28;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Post-tetanic potentiation in Aplysia sensory neurons. Brain Res. 1984 Feb 20;293(2):377–380. doi: 10.1016/0006-8993(84)91247-2. [DOI] [PubMed] [Google Scholar]


