Abstract
Fluorescence recovery after photobleaching was used to determine the diffusion coefficients of the oxidation-reduction (redox) components ubiquinone, complex III (cytochromes b-c1), cytochrome c, and complex IV (cytochrome oxidase) of the mitochondrial inner membrane. All redox components diffuse in two dimensions as common-pool electron carriers. Cytochrome c diffuses in two and three dimensions concomitantly, and its diffusion rate, unlike that of all other redox components, is modulated along with its activity by ionic strength. The diffusion coefficients established in this study reveal that the theoretical diffusion-controlled collision frequencies of all redox components are greater than their experimental maximum (uncoupled) turnover numbers. Since electron transport is slower than the theoretical limit set by the lateral diffusion of the redox components, ordered chains, assemblies, or aggregates of redox components are not necessary to account for electron transport. Rather, mitochondrial electron transport is diffusion coupled, consistent with a "random-collision model" for electron transport.
Full text
PDF
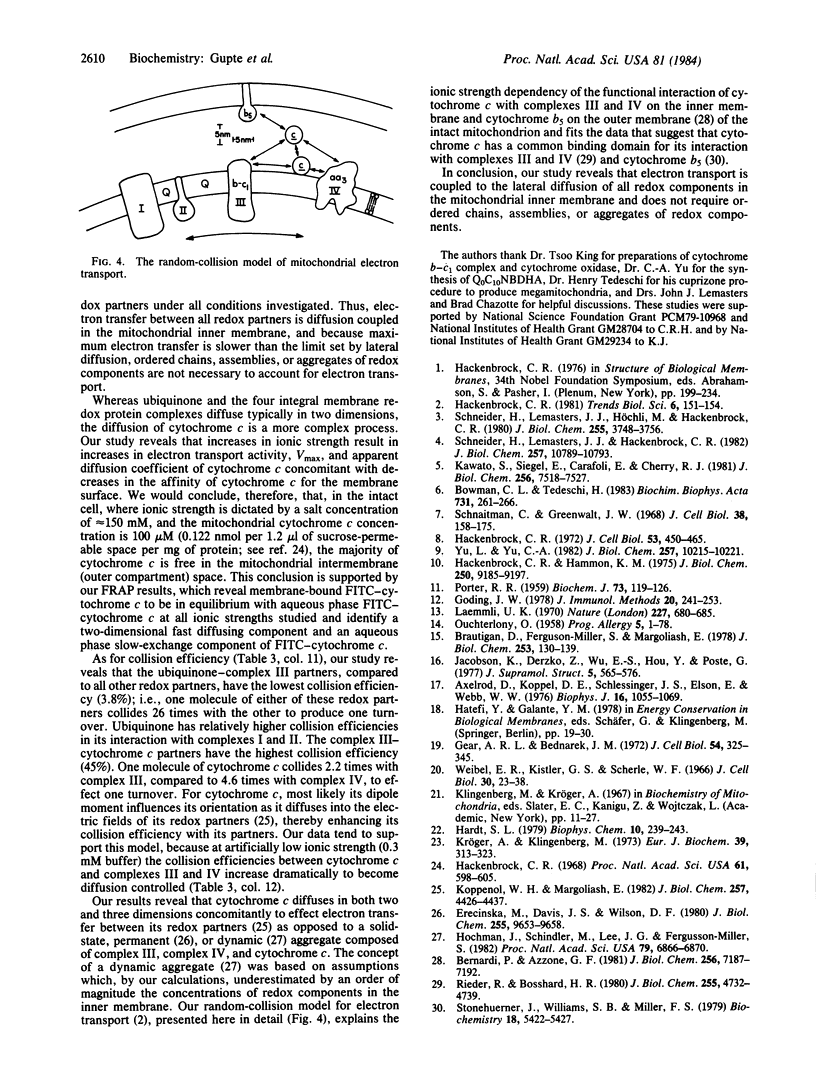
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P., Azzone G. F. Cytochrome c as an electron shuttle between the outer and inner mitochondrial membranes. J Biol Chem. 1981 Jul 25;256(14):7187–7192. [PubMed] [Google Scholar]
- Bowman C. L., Tedeschi H. Kinetics of Lucifer yellow CH efflux in giant mitochondria. Biochim Biophys Acta. 1983 Jun 10;731(2):261–266. doi: 10.1016/0005-2736(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Definition of cytochrome c binding domains by chemical modification. I. Reaction with 4-chloro-3,5-dinitrobenzoate and chromatographic separation of singly substituted derivatives. J Biol Chem. 1978 Jan 10;253(1):130–139. [PubMed] [Google Scholar]
- Erecińska M., Davis J. S., Wilson D. F. Interactions of cytochrome c with mitochondrial membranes. Binding to succinate-cytochrome c reductase. J Biol Chem. 1980 Oct 25;255(20):9653–9658. [PubMed] [Google Scholar]
- Gear A. R., Bednarek J. M. Direct counting and sizing of mitochondria in solution. J Cell Biol. 1972 Aug;54(2):325–345. doi: 10.1083/jcb.54.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci U S A. 1968 Oct;61(2):598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Energy-linked ultrastructural transformations in isolated liver mitochondria and mitoplasts. Preservation of configurations by freeze-cleaving compared to chemical fixation. J Cell Biol. 1972 May;53(2):450–465. doi: 10.1083/jcb.53.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt S. L. Rates of diffusion controlled reactions in one, two and three dimensions. Biophys Chem. 1979 Nov;10(3-4):239–243. doi: 10.1016/0301-4622(79)85012-7. [DOI] [PubMed] [Google Scholar]
- Hochman J. H., Schindler M., Lee J. G., Ferguson-Miller S. Lateral mobility of cytochrome c on intact mitochondrial membranes as determined by fluorescence redistribution after photobleaching. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6866–6870. doi: 10.1073/pnas.79.22.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Kawato S., Sigel E., Carafoli E., Cherry R. J. Rotation of cytochrome oxidase in phospholipid vesicles. Investigations of interactions between cytochrome oxidases and between cytochrome oxidase and cytochrome bc1 complex. J Biol Chem. 1981 Jul 25;256(14):7518–7527. [PubMed] [Google Scholar]
- Koppenol W. H., Margoliash E. The asymmetric distribution of charges on the surface of horse cytochrome c. Functional implications. J Biol Chem. 1982 Apr 25;257(8):4426–4437. [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem. 1973 Nov 15;39(2):313–323. doi: 10.1111/j.1432-1033.1973.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. Comparison of the binding sites on cytochrome c for cytochrome c oxidase, cytochrome bc1, and cytochrome c1. Differential acetylation of lysyl residues in free and complexed cytochrome c. J Biol Chem. 1980 May 25;255(10):4732–4739. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Lemasters J. J., Hackenbrock C. R. Lateral diffusion of ubiquinone during electron transfer in phospholipid- and ubiquinone-enriched mitochondrial membranes. J Biol Chem. 1982 Sep 25;257(18):10789–10793. [PubMed] [Google Scholar]
- Schneider H., Lemasters J. J., Höchli M., Hackenbrock C. R. Liposome-mitochondrial inner membrane fusion. Lateral diffusion of integral electron transfer components. J Biol Chem. 1980 Apr 25;255(8):3748–3756. [PubMed] [Google Scholar]
- Stonehuerner J., Williams J. B., Millett F. Interaction between cytochrome c and cytochrome b5. Biochemistry. 1979 Nov 27;18(24):5422–5427. doi: 10.1021/bi00591a026. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Yu C. A. The interaction of arylazido ubiquinone derivative with mitochondrial ubiquinol-cytochrome c reductase. J Biol Chem. 1982 Sep 10;257(17):10215–10221. [PubMed] [Google Scholar]