Abstract
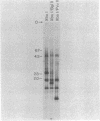
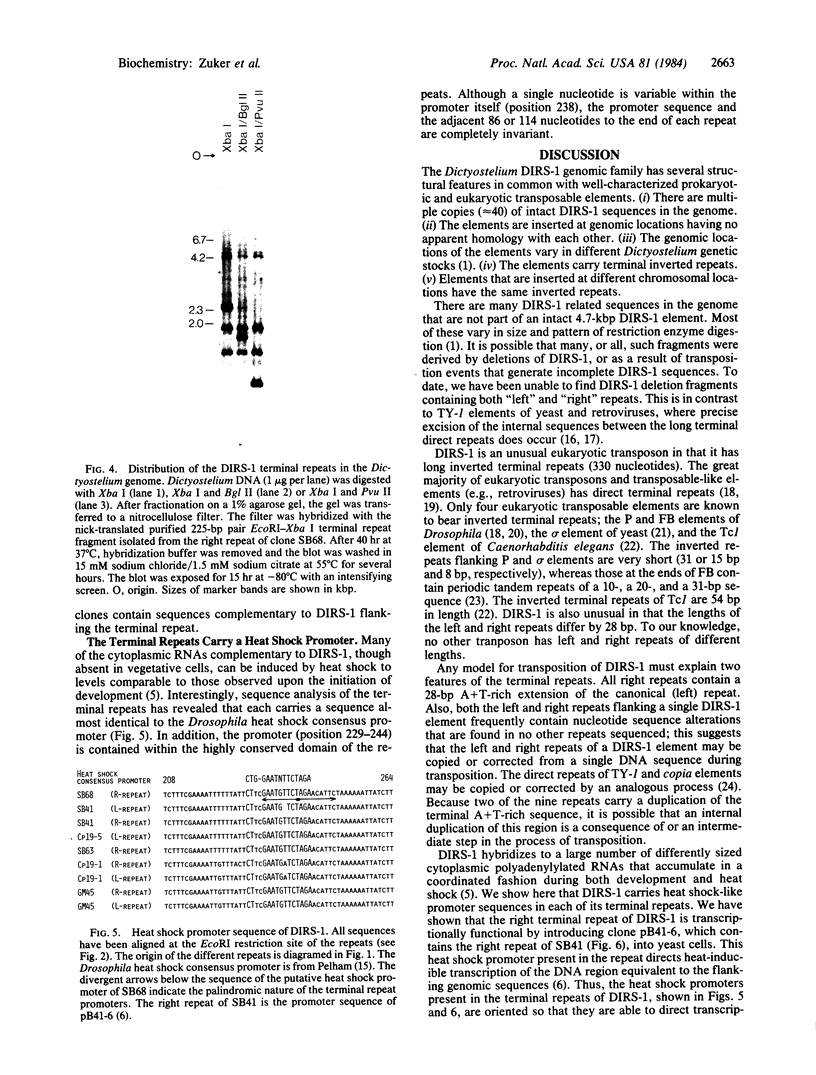
DIRS-1 is a 4.7-kilobase-pair repetitive and apparently transposable Dictyostelium genetic element that is transcribed during differentiation or after heat shock. The terminal regions of DIRS-1 are inverted repeats of 330 base pairs. The repeats are highly conserved both within a given element as well as between different members of the family (less than 10% divergence). At the distal end of all left repeats is a 32-nucleotide sequence composed almost entirely of A and T residues. In addition to this 32-base A + T sequence, the distal region of all right repeats is extended by a 28-base-pair A + T-rich sequence that is identical in all copies. The sequences flanking each DIRS-1 sequence are completely dissimilar, and there appears to be no duplication of the genomic DNA sequence at the presumed point of DIRS-1 insertion. The terminal repeats can also be found interspersed in the genome independently of the complete element. In addition, the terminal repeats carry a 15-nucleotide sequence that greatly resembles the Drosophila consensus heat shock promoter and may be involved in the transcriptional induction of the DIRS-1 sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Loh E. Y., Davis R. W. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell. 1979 Apr;16(4):739–751. doi: 10.1016/0092-8674(79)90090-4. [DOI] [PubMed] [Google Scholar]
- Cappello J., Zuker C., Lodish H. F. Repetitive Dictyostelium heat-shock promotor functions in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Apr;4(4):591–598. doi: 10.1128/mcb.4.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Zuker C., Lodish H. F. A repetitive and apparently transposable DNA sequence in Dictyostelium discoideum associated with developmentally regulated RNAs. Nucleic Acids Res. 1983 Jul 25;11(14):4835–4852. doi: 10.1093/nar/11.14.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Eibel H., Gafner J., Stotz A., Philippsen P. Characterization of the yeast mobile element Ty1. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):609–617. doi: 10.1101/sqb.1981.045.01.079. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature. 1980 Jul 24;286(5771):352–356. doi: 10.1038/286352a0. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Levis R., Dunsmuir P., Rubin G. M. Terminal repeats of the Drosophila transposable element copia: nucleotide sequence and genomic organization. Cell. 1980 Sep;21(2):581–588. doi: 10.1016/0092-8674(80)90496-1. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Swanstrom R., DeLorbe W. J., Payne G. S., Hughes S. H., Ortiz S., Quintrell N., Bishop J. M., Varmus H. E. DNA intermediates in the replication of retroviruses are structurally (and perhaps functionally) related to transposable elements. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):731–738. doi: 10.1101/sqb.1981.045.01.091. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Chung S., Zuker C., Lodish H. F. Selection and analysis of cloned developmentally-regulated Dictyostelium discoideum genes by hybridization-competition. Nucleic Acids Res. 1981 Feb 25;9(4):947–963. doi: 10.1093/nar/9.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Potter S. S. DNA sequence of a foldback transposable element in Drosophila. Nature. 1982 May 20;297(5863):201–204. doi: 10.1038/297201a0. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Target sequences for the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Oct 25;11(20):7137–7140. doi: 10.1093/nar/11.20.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Drosophila genome organization: conserved and dynamic aspects. Annu Rev Genet. 1981;15:219–264. doi: 10.1146/annurev.ge.15.120181.001251. [DOI] [PubMed] [Google Scholar]
- Zuker C., Cappello J., Chisholm R. L., Lodish H. F. A repetitive Dictyostelium gene family that is induced during differentiation and by heat shock. Cell. 1983 Oct;34(3):997–1005. doi: 10.1016/0092-8674(83)90557-3. [DOI] [PubMed] [Google Scholar]
- Zuker C., Lodish H. F. Repetitive DNA sequences cotranscribed with developmentally regulated Dictyostelium discoideum mRNAs. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5386–5390. doi: 10.1073/pnas.78.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey F. J., Donahue T. F., Fink G. R. sigma, a repetitive element found adjacent to tRNA genes of yeast. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4138–4142. doi: 10.1073/pnas.79.13.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]