Abstract
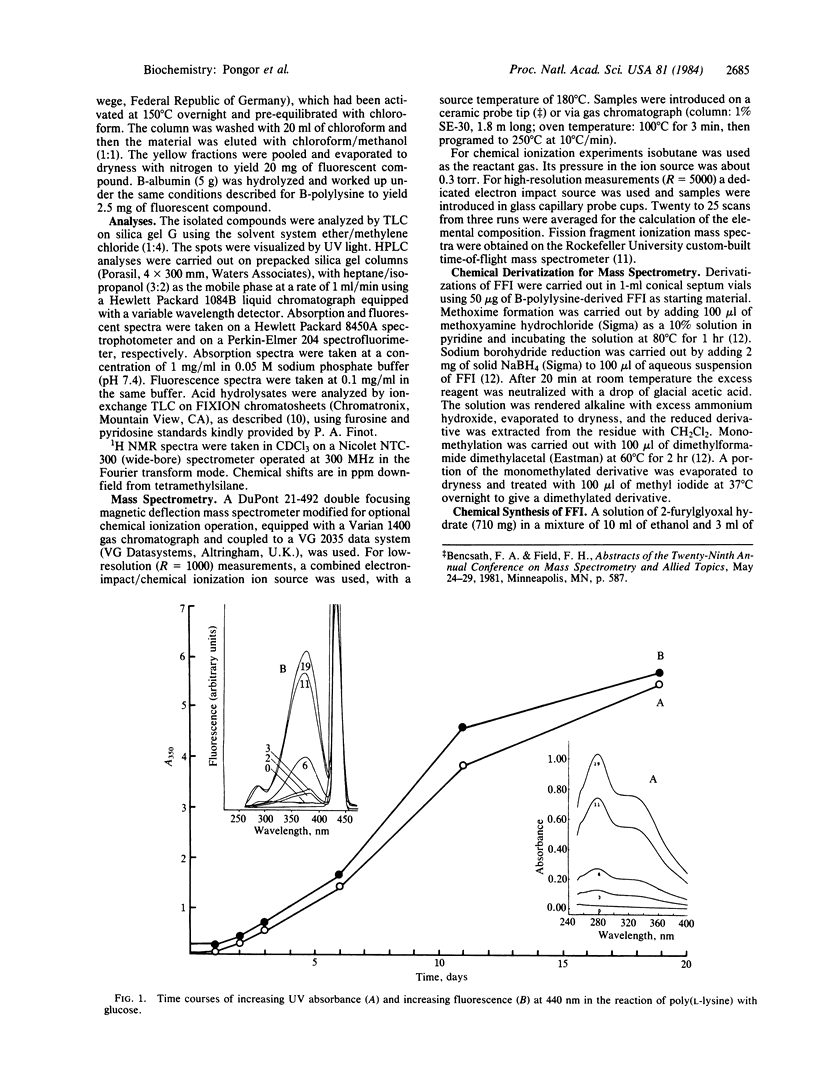
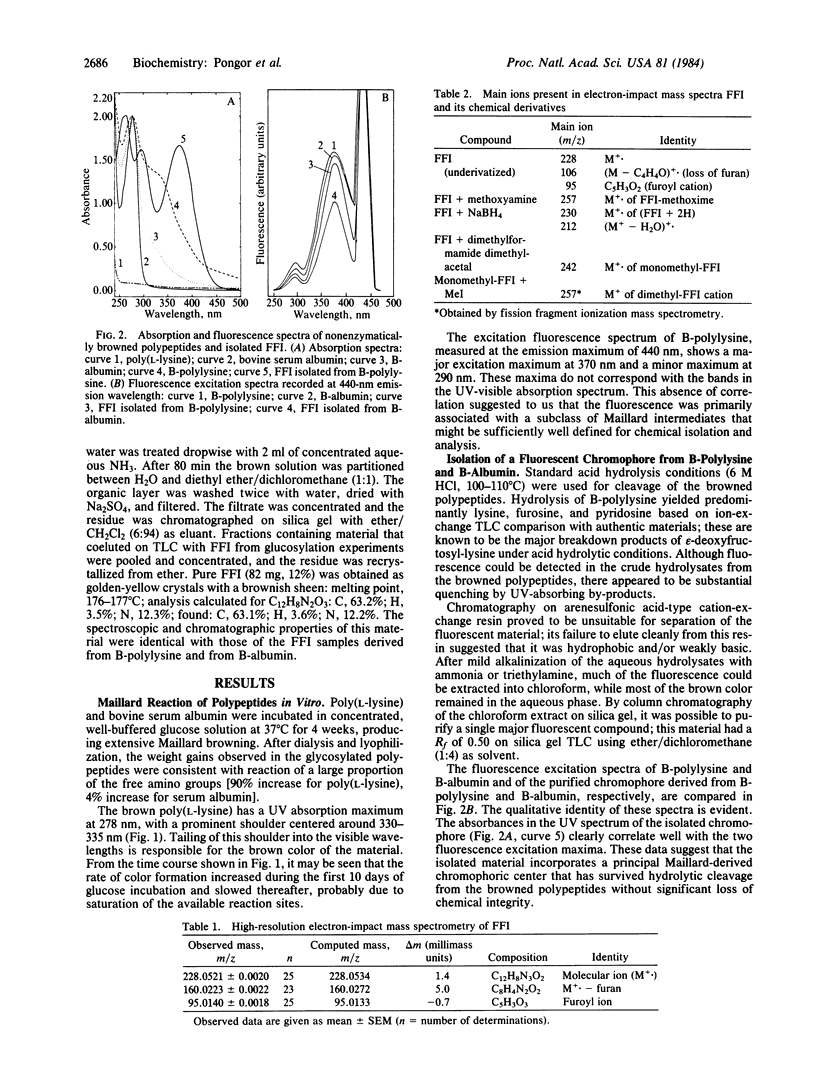
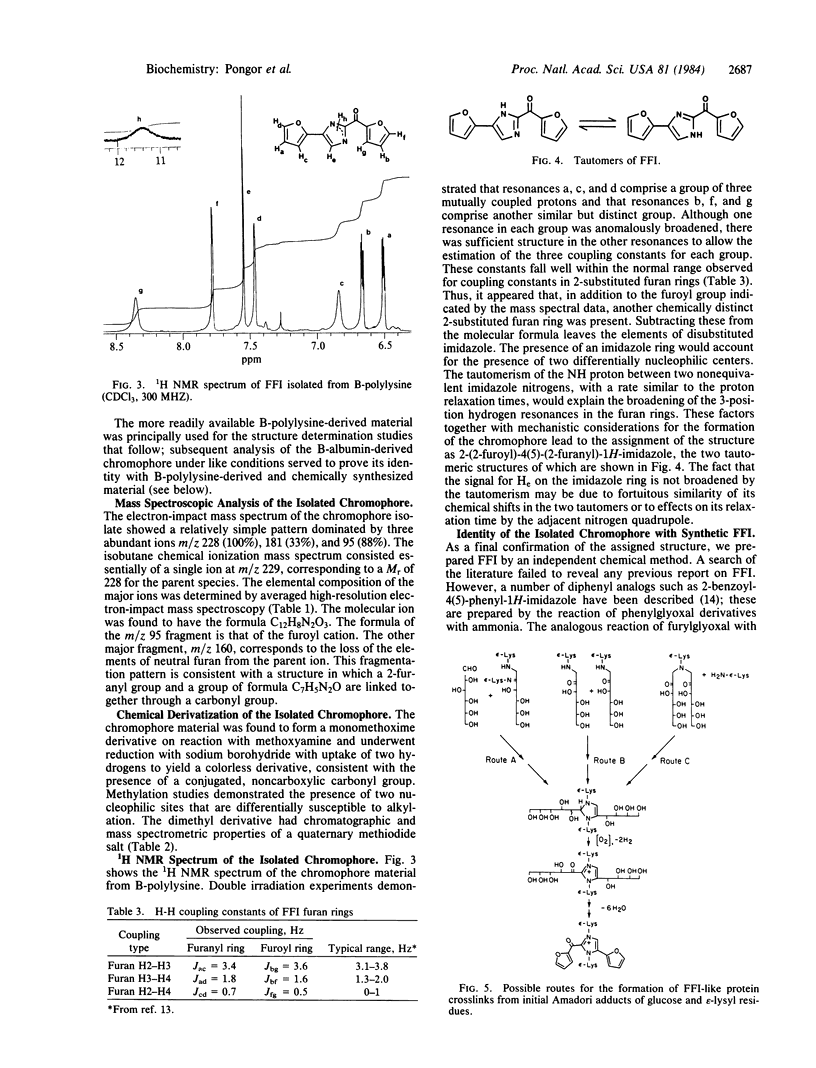
Proteins exposed to glucose over long periods are known to undergo physicochemical changes including crosslinking and formation of brown fluorescent pigments of poorly characterized structure. Acid hydrolysis of both browned poly(L-lysine) and browned bovine serum albumin is found to release a major fluorescent chromophore, which after alkalinization is extractable into organic solvents and which can be purified by silica gel chromatography. The fluorescence properties of this compound very closely resemble those of the bulk browned polypeptides. By NMR, mass spectroscopy, and chemical derivatization, this compound is assigned the structure 2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole (FFI). Confirmation was obtained by independent chemical synthesis from furylglyoxal and ammonia. The incorporation of two peptide-derived amine nitrogens and two glucose residues in FFI strongly suggests that peptide-bound FFI precursors are implicated in the crosslinking of proteins by glucose in vivo. This reaction has potential implications in the understanding of glucose-mediated protein modifications and their role in the complications of diabetes and aging.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee M., Pongor S., Cerami A. Covalent attachment of soluble proteins by nonenzymatically glycosylated collagen. Role in the in situ formation of immune complexes. J Exp Med. 1983 Nov 1;158(5):1739–1744. doi: 10.1084/jem.158.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Model P., Cerami A. Modification of DNA by reducing sugars: a possible mechanism for nucleic acid aging and age-related dysfunction in gene expression. Proc Natl Acad Sci U S A. 1984 Jan;81(1):105–109. doi: 10.1073/pnas.81.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Gabbay K. H., Gallop P. M. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem Biophys Res Commun. 1975 Nov 3;67(1):103–109. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Blobstein S. H., Cerami A. Structure of carbohydrate of hemoglobin AIc. J Biol Chem. 1977 May 10;252(9):2992–2997. [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Detection of nonenzymatic browning products in the human lens. Biochim Biophys Acta. 1983 Oct 4;760(1):97–103. doi: 10.1016/0304-4165(83)90129-0. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Kohn R. R., Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]



