Abstract
Transferrin (Tf) is the major iron binding protein in vertebrate serum. It shares homologous amino acid sequences with four other proteins: lactotransferrin, ovotransferrin, melanoma antigen p97, and HuBlym-1. Antigen p97 and the Tf receptor genes have been mapped on human chromosome 3. The goal of the study described here was to initiate the characterization of the Tf gene by identifying and characterizing its cDNA and mapping its chromosomal location. Recombinant plasmids containing human cDNA encoding Tf have been isolated by screening an adult human liver library with a mixed oligonucleotide probe. Within the 2.3 kilobase pairs of Tf cDNA analyzed, there is a probable leader sequence encoded by 57 nucleotides followed by 2037 nucleotides that encode the homologous amino and carboxyl domains. During evolution, three areas of the homologous amino and carboxyl domains have been strongly conserved, possibly reflecting functional constraints associated with iron binding. Chromosomal mapping by in situ hybridization and somatic cell hybrid analysis indicate that the Tf gene is located at q21-25 on human chromosome 3, consistent with linkage of the Tf, Tf receptor, and melanoma p97 loci.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado-Urbina G., Sathe G. M., Liu W. C., Gillen M. F., Duck P. D., Bender R., Ogilvie K. K. Automated synthesis of gene fragments. Science. 1981 Oct 16;214(4518):270–274. doi: 10.1126/science.6169150. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Hewick R. M., Hellström I., Hellström K. E., Doolittle R. F., Dreyer W. J. Human melanoma-associated antigen p97 is structurally and functionally related to transferrin. Nature. 1982 Mar 11;296(5853):171–173. doi: 10.1038/296171a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Diamond A., Cooper G. M., Ritz J., Lane M. A. Identification and molecular cloning of the human Blym transforming gene activated in Burkitt's lymphomas. Nature. 1983 Sep 8;305(5930):112–116. doi: 10.1038/305112a0. [DOI] [PubMed] [Google Scholar]
- Enns C. A., Suomalainen H. A., Gebhardt J. E., Schröder J., Sussman H. H. Human transferrin receptor: expression of the receptor is assigned to chromosome 3. Proc Natl Acad Sci U S A. 1982 May;79(10):3241–3245. doi: 10.1073/pnas.79.10.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Sutherland R., Greaves M., Solomon E., Povey S. Expression of human transferrin receptor is controlled by a gene on chromosome 3: assignment using species specificity of a monoclonal antibody. Somatic Cell Genet. 1982 Mar;8(2):197–206. doi: 10.1007/BF01538677. [DOI] [PubMed] [Google Scholar]
- Goubin G., Goldman D. S., Luce J., Neiman P. E., Cooper G. M. Molecular cloning and nucleotide sequence of a transforming gene detected by transfection of chicken B-cell lymphoma DNA. Nature. 1983 Mar 10;302(5904):114–119. doi: 10.1038/302114a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
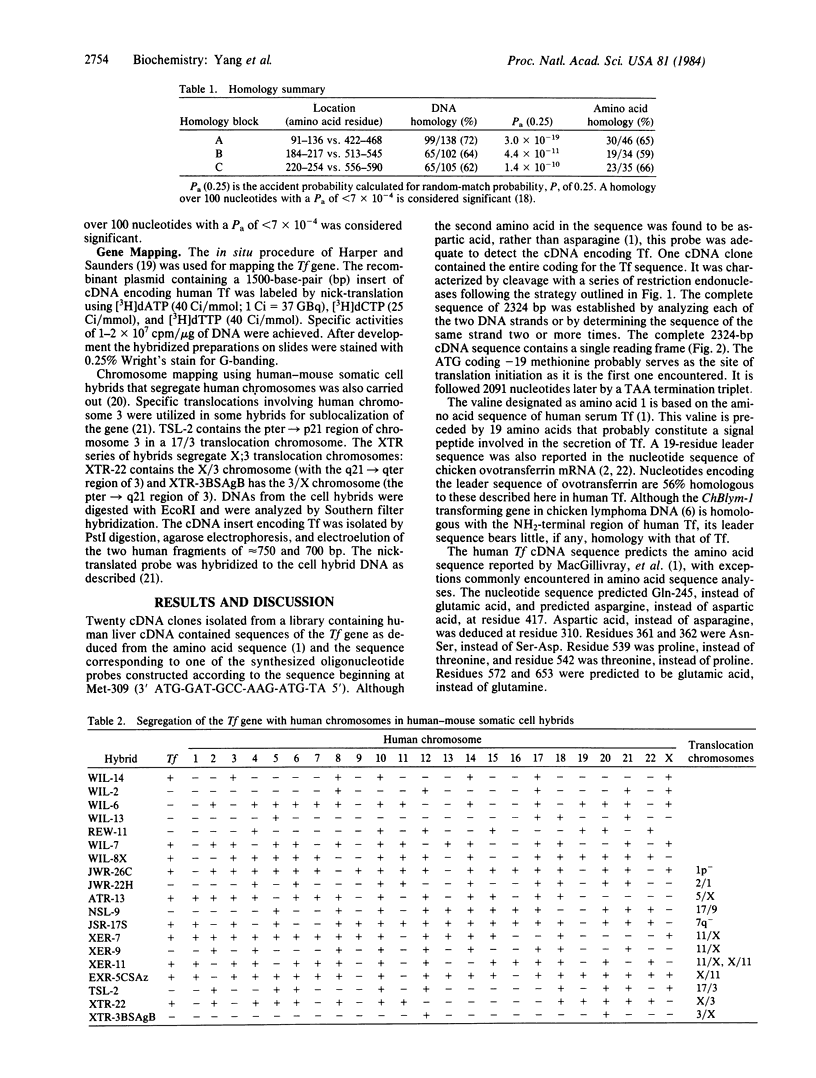
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Jeltsch J. M., Chambon P. The complete nucleotide sequence of the chicken ovotransferrin mRNA. Eur J Biochem. 1982 Feb;122(2):291–295. doi: 10.1111/j.1432-1033.1982.tb05879.x. [DOI] [PubMed] [Google Scholar]
- MacGillivray R. T., Mendez E., Shewale J. G., Sinha S. K., Lineback-Zins J., Brew K. The primary structure of human serum transferrin. The structures of seven cyanogen bromide fragments and the assembly of the complete structure. J Biol Chem. 1983 Mar 25;258(6):3543–3553. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Conneally P. M. Report of the Committee on Unassigned Linkage Groups. Cytogenet Cell Genet. 1984;37(1-4):205–209. doi: 10.1159/000132010. [DOI] [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Mazurier J., Jollès J., Spik G., Montreuil J., Jollès P. The present state of the human lactotransferrin sequence. Study and alignment of the cyanogen bromide fragments and characterization of N- and C-terminal domains. Biochim Biophys Acta. 1981 Sep 29;670(2):243–254. doi: 10.1016/0005-2795(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Miller Y. E., Jones C., Scoggin C., Morse H., Seligman P. Chromosome 3q (22-ter) encodes the human transferrin receptor. Am J Hum Genet. 1983 Jul;35(4):573–583. [PMC free article] [PubMed] [Google Scholar]
- Naylor S. L., Elliott R. W., Brown J. A., Shows T. B. Mapping of aminoacylase-1 and beta-galactosidase-A to homologous regions of human chromosome 3 and mouse chromosome 9 suggests location of additional genes. Am J Hum Genet. 1982 Mar;34(2):235–244. [PMC free article] [PubMed] [Google Scholar]
- Naylor S. L., Elliott R. W., Brown J. A., Shows T. B. Mapping of aminoacylase-1 and beta-galactosidase-A to homologous regions of human chromosome 3 and mouse chromosome 9 suggests location of additional genes. Am J Hum Genet. 1982 Mar;34(2):235–244. [PMC free article] [PubMed] [Google Scholar]
- Plowman G. D., Brown J. P., Enns C. A., Schröder J., Nikinmaa B., Sussman H. H., Hellström K. E., Hellström I. Assignment of the gene for human melanoma-associated antigen p97 to chromosome 3. Nature. 1983 May 5;303(5912):70–72. doi: 10.1038/303070a0. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Markham A. F., Orkin S. H. Isolation of a cDNA clone for human antithrombin III. J Biol Chem. 1983 Jul 10;258(13):8389–8394. [PubMed] [Google Scholar]
- SMITHIES O., CONNELL G. E., DIXON G. H. Chromosomal rearrangements and the evolution of haptoglobin genes. Nature. 1962 Oct 20;196:232–236. doi: 10.1038/196232a0. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Yang M., Bonner J. Nucleotide sequence of cloned rat serum albumin messenger RNA. Proc Natl Acad Sci U S A. 1981 Jan;78(1):243–246. doi: 10.1073/pnas.78.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau S. N., Lee D. C., Palmiter R. D. Identical precursors for serum transferrin and egg white conalbumin. J Biol Chem. 1978 Jun 10;253(11):3771–3774. [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. C., Sutton H. E., Howard P. N. Human transferrins C and D-Chi: an amino acid difference. Biochem Genet. 1967 Jun;1(1):55–59. doi: 10.1007/BF00487736. [DOI] [PubMed] [Google Scholar]
- Wang A. C., Sutton H. E. Human Transferrins C and D1: Chemical Difference in a Peptide. Science. 1965 Jul 23;149(3682):435–437. doi: 10.1126/science.149.3682.435. [DOI] [PubMed] [Google Scholar]
- Wang A. C., Sutton H. E., Riggs A. A chemical difference between human transferrins B2 and C. Am J Hum Genet. 1966 Sep;18(5):454–458. [PMC free article] [PubMed] [Google Scholar]
- Williams J., Elleman T. C., Kingston I. B., Wilkins A. G., Kuhn K. A. The primary structure of hen ovotransferrin. Eur J Biochem. 1982 Feb;122(2):297–303. doi: 10.1111/j.1432-1033.1982.tb05880.x. [DOI] [PubMed] [Google Scholar]
- Yang F., Brune J. L., Baldwin W. D., Barnett D. R., Bowman B. H. Identification and characterization of human haptoglobin cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5875–5879. doi: 10.1073/pnas.80.19.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]