Abstract
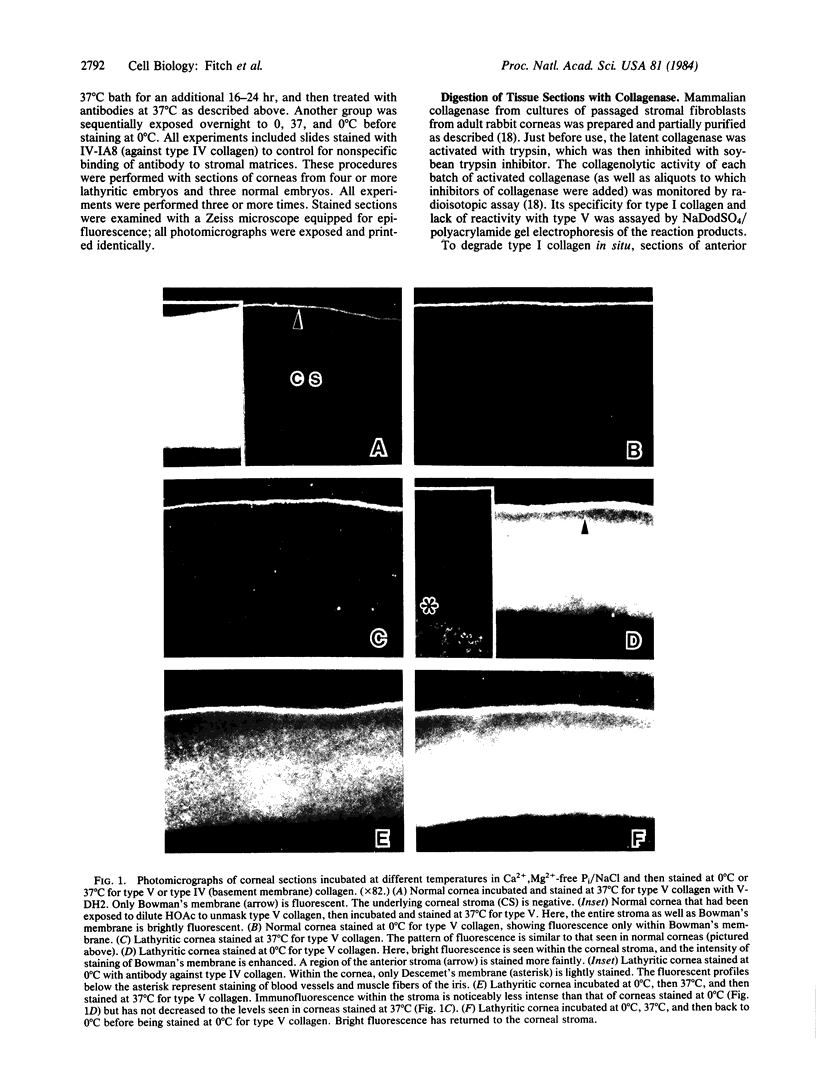
To determine whether type V collagen is antigenically masked in situ by its fibrillar organization, two different methods were used to perturb selectively the structure of collagen fibrils in sections of embryonic chicken corneas. The experimentally modified tissues were probed by immunohistochemical procedures with monoclonal antibodies against types V and I. A lathyritic agent was used to block crosslinking of newly synthesized collagen. This results in reversible temperature-sensitive alterations in fibrillar packing, such that freshly formed collagen fibrils retain their aggregated state at 37 degrees C but become dissociated upon cooling. Type V-specific immunofluorescence remained masked at 37 degrees C but was revealed at 0 degree C. The effect of temperature was partially reversible, indicating that type V collagen is normally unavailable for antibody binding because of its fibrillar arrangement. In sections of normal corneas, treatment with corneal collagenase, which degrades type I collagen, but not type V, also unmasked the latter. This implicates type I collagen as the masking agent. We propose that collagen types I and V are incorporated together in heterotypic fibrils.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davison P. F., Hong B. S., Cannon D. J. Quantitative analysis of the collagens in the bovine cornea. Exp Eye Res. 1979 Aug;29(2):97–107. doi: 10.1016/0014-4835(79)90075-7. [DOI] [PubMed] [Google Scholar]
- Fitch J. M., Gibney E., Sanderson R. D., Mayne R., Linsenmayer T. F. Domain and basement membrane specificity of a monoclonal antibody against chicken type IV collagen. J Cell Biol. 1982 Nov;95(2 Pt 1):641–647. doi: 10.1083/jcb.95.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix M. J., Hay E. D., von der Mark K., Linsenmayer T. F. Immunohistochemical localization of collagen types I and II in the developing chick cornea and tibia by electron microscopy. Invest Ophthalmol Vis Sci. 1982 Mar;22(3):359–375. [PubMed] [Google Scholar]
- Henkel W., Glanville R. W. Covalent crosslinking between molecules of type I and type III collagen. The involvement of the N-terminal, nonhelical regions of the alpha 1 (I) and alpha 1 (III) chains in the formation of intermolecular crosslinks. Eur J Biochem. 1982 Feb;122(1):205–213. doi: 10.1111/j.1432-1033.1982.tb05868.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Wint B. A quantitative collagen film collagenase assay for large numbers of samples. Anal Biochem. 1980 May 1;104(1):175–181. doi: 10.1016/0003-2697(80)90295-x. [DOI] [PubMed] [Google Scholar]
- LEVENE C. I., GROSS J. Alterations in state of molecular aggregation of collagen induced in chick embryos by beta-aminopropionitrile (lathyrus factor). J Exp Med. 1959 Nov 1;110:771–790. doi: 10.1084/jem.110.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer T. F., Fitch J. M., Mayne R. Extracellular matrices in the developing avian eye: type V collagen in corneal and noncorneal tissues. Invest Ophthalmol Vis Sci. 1984 Jan;25(1):41–47. [PubMed] [Google Scholar]
- Linsenmayer T. F., Fitch J. M., Schmid T. M., Zak N. B., Gibney E., Sanderson R. D., Mayne R. Monoclonal antibodies against chicken type V collagen: production, specificity, and use for immunocytochemical localization in embryonic cornea and other organs. J Cell Biol. 1983 Jan;96(1):124–132. doi: 10.1083/jcb.96.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer T. F., Hendrix M. J., Little C. D. Production and characterization of a monoclonal antibody to chicken type I collagen. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3703–3707. doi: 10.1073/pnas.76.8.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöschl A., von der Mark K. Synthesis of type V collagen by chick corneal fibroblasts in vivo and in vitro. FEBS Lett. 1980 Jun 16;115(1):100–104. doi: 10.1016/0014-5793(80)80735-6. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L. Cross-linking of collagen. Science. 1973 May 11;180(4086):561–566. doi: 10.1126/science.180.4086.561. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Smuckler D., Stern R. Comparison of collagen types in adult and fetal bovine corneas. J Biol Chem. 1982 Mar 10;257(5):2627–2633. [PubMed] [Google Scholar]
- VAN DEN HOOFF A., LEVENE C. I., GROSS J. Morphologic evidence for collagen changes in chick embryos treated with beta-aminopropionitrile. J Exp Med. 1959 Dec 1;110:1017–1022. doi: 10.1084/jem.110.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh C., Gay S., Rhodes R. K., Pfister R., Miller E. J. Collagen heterogeneity in normal rabbit cornea. I. Isolation and biochemical characterization of the genetically-distinct collagens. Biochim Biophys Acta. 1980 Sep 23;625(1):78–88. doi: 10.1016/0005-2795(80)90110-5. [DOI] [PubMed] [Google Scholar]





