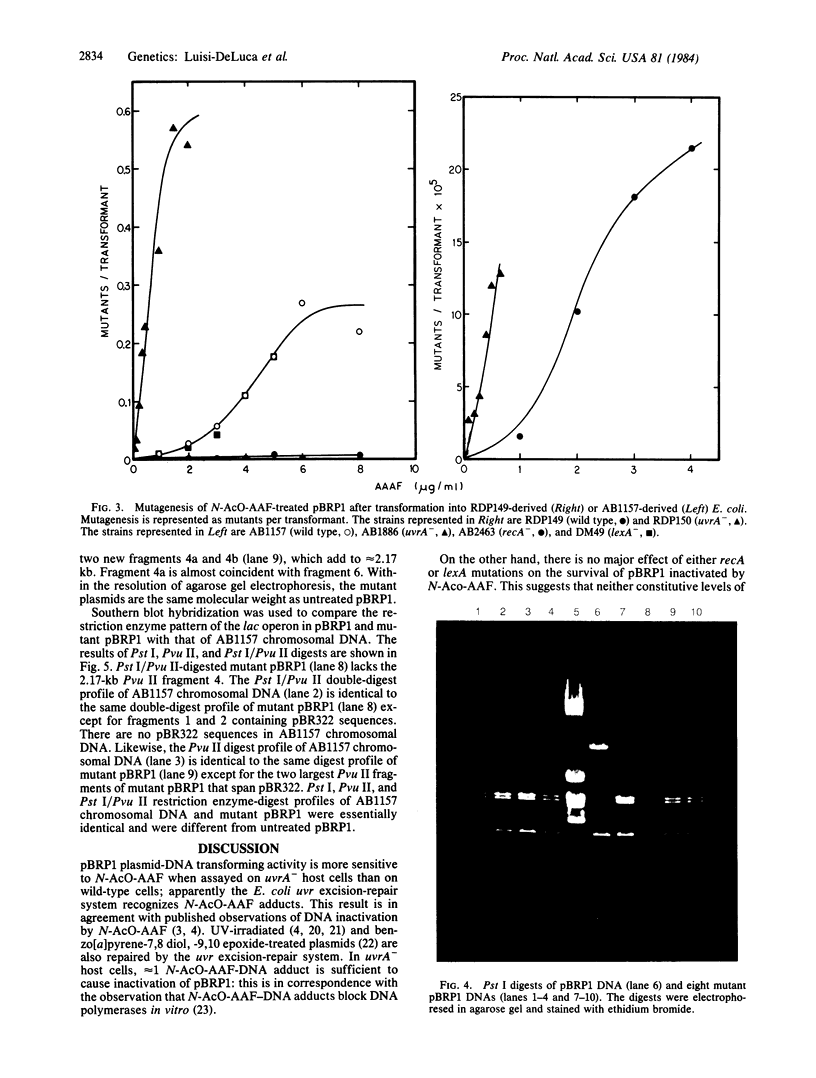
Abstract
A plasmid containing a wild-type lac operon and a tetracycline-resistance gene was covalently modified by N-acetoxy-2-acetylaminofluorene and used to transform two series of Lac- Escherichia coli cell types. Each set contained wild-type and repair-deficient mutants. One set of cells contained a lacY mutation and the other a deletion of the entire lac operon. Survival and mutagenesis of the plasmid were measured as a function of the N-acetoxy-2-acetylaminofluorene concentration. The results indicate that when no homologous sequences are present in the chromosomal DNA, mutations occur at a low frequency: at 10% survival the frequency was 1-2 X 10(-4) mutants per transformant. When homologous sequences, the lacY allele, are present in the chromosomal DNA, Lac- plasmids are found at a high frequency in a recA-dependent, lexA-independent fashion: at 10% survival the frequency was 5-10 X 10(-2) mutants per transformant. Southern blot analysis of the restriction enzyme profiles of the resulting plasmid and host-cell DNA sequences showed recombinational transfer of host sequences to the N-acetoxy-2-acetylamino-fluorene-treated plasmid had occurred. When the host chromosomes contained Lac+ homologous sequences no mutants were found, indicating that the results were not caused by error-prone recombination.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Chaconas G., de Bruijn F. J., Casadaban M. J., Lupski J. R., Kwoh T. J., Harshey R. M., DuBow M. S., Bukhari A. I. In vitro and in vivo manipulations of bacteriophage Mu DNA: cloning of Mu ends and construction of mini-Mu's carrying selectable markers. Gene. 1981 Jan-Feb;13(1):37–46. doi: 10.1016/0378-1119(81)90041-x. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Low B. Some genetic consequences of changes in the level of recA gene function in Escherichia coli K-12. Genetics. 1976 Dec;84(4):675–695. doi: 10.1093/genetics/84.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy M. H. Frameshift mutations in the lactose operon of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:189–201. doi: 10.1101/sqb.1966.031.01.027. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa H., Lee C. H., Kakefuda T. Alteration of plasmid DNA-mediated transformation and mutation induced by covalent binding of benzo[alpha]pyrene-7,8-dihydrodiol-9,10-oxide in Escherichia coli. Mutat Res. 1981 Jun;82(1):47–57. doi: 10.1016/0027-5107(81)90137-8. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M., Jeggo P., Wagner R. Chromosomal rearrangement and carcinogenesis. Mutat Res. 1982 May;98(3):249–264. doi: 10.1016/0165-1110(82)90035-5. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Strike P. Efficiency of Escherichia coli repair processes on uv-damaged transforming plasmid DNA. Plasmid. 1981 Mar;5(2):213–220. doi: 10.1016/0147-619x(81)90022-6. [DOI] [PubMed] [Google Scholar]
- Schmid S. E., Daune M. P., Fuchs R. P. Repair and mutagenesis of plasmid DNA modified by ultraviolet irradiation or N-acetoxy-N-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4133–4137. doi: 10.1073/pnas.79.13.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]