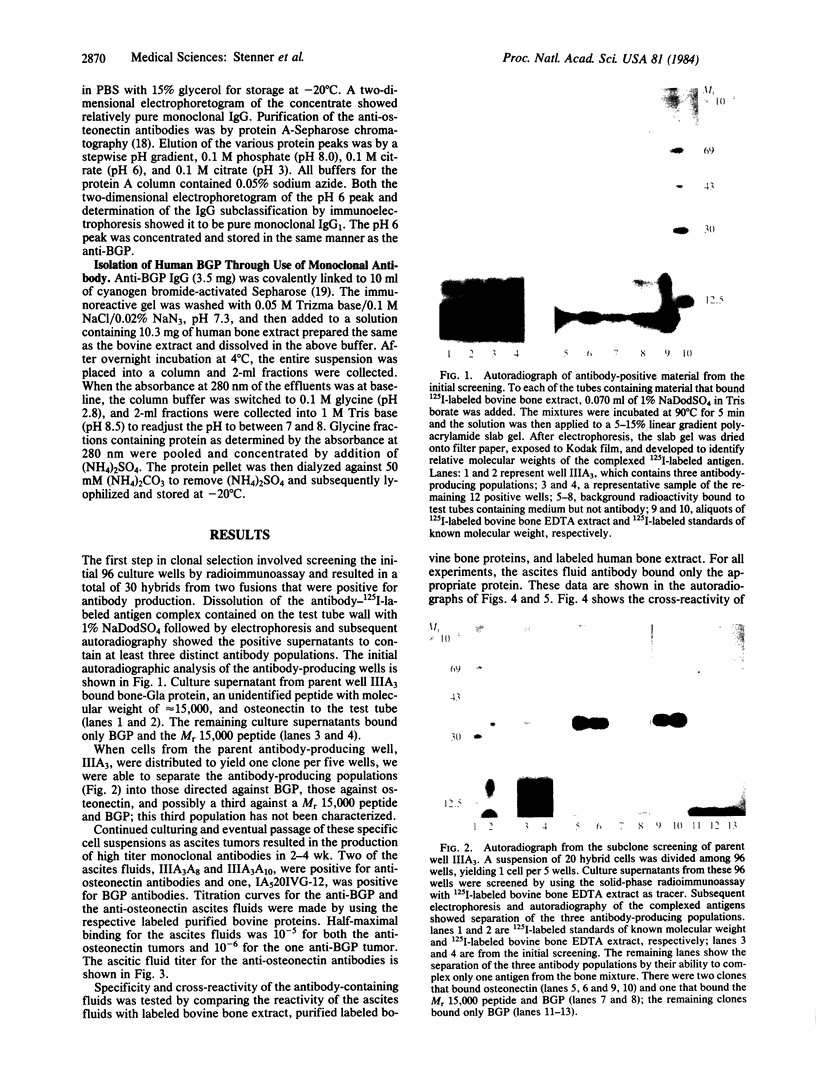
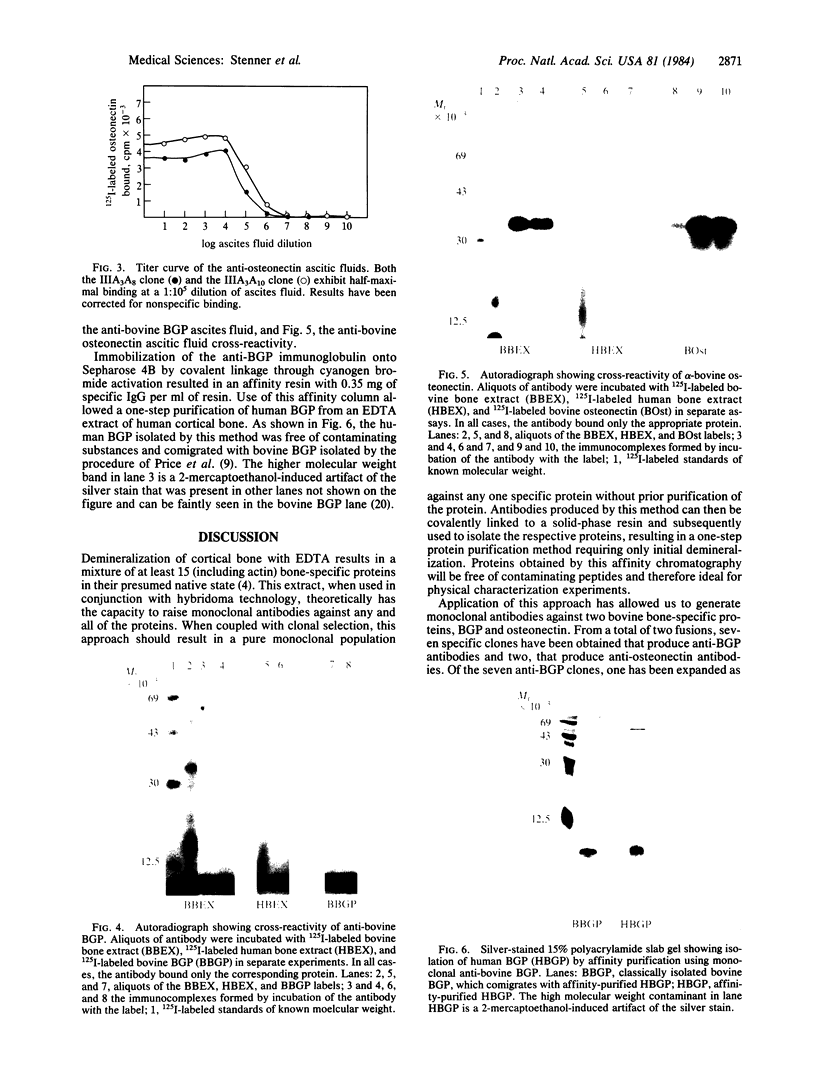
Abstract
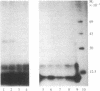
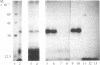
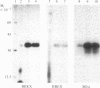
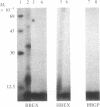
Hybridoma technology was used for preparation of murine monoclonal antibodies of high titer against bone-Gla protein and osteonectin. A procedure of immunization and hybridization similar to that already described [Katzmann, J.A., Nesheim, M.E., Hibbard, L.S. & Mann, K.G. (1981) Proc. Natl. Acad. Sci. USA 78, 162-166; and Foster, W.B., Katzmann, J.A., Miller, R.S., Nesheim, M.E. & Mann, K.G. (1982) Thromb. Res. 28, 649-661] was used. However, in contrast to earlier studies, mice were immunized with an unfractionated protein mixture that had been extracted from bone under nondenaturing conditions. The extract was labeled with 125I by the chloramine-T method. After fusion and initial hybrid growth, screening was accomplished by a solid-phase radioimmunoassay with total 125I-labeled bovine bone protein extract as the tracer. The identities of antibody-bound 125I-labeled proteins were assessed by dissolution of the solid-phase immune complex in NaDodSO4 and subsequent electrophoresis and autoradiography. Clones producing specific antibody to a single protein were selected by limiting dilution. The identity of the proteins against which the specific antibodies were produced was confirmed by immunoprecipitation, electrophoresis, and autoradiography. From two fusions, 30 positive hybrids to bone-Gla protein were identified; 7 of these were subcloned and 1 has been expanded as an ascites tumor. One hybrid population was positive for osteonectin, a Mr 15,000 peptide, and for bone-Gla protein. By limiting dilution, the osteonectin clone was selected and subsequently expanded as an ascites tumor. Titration curves made using the respective 125I-labeled purified proteins show the ascites tumors to be producing antibody of high titer (I50 = 10(-6) for anti-bone-Gla protein and (I50 = 10(-5) for antiosteonectin. Both of the antibovine antibodies are cross-reactive with the corresponding human protein. Immobilized specific anti-bone-Gla protein has been used to isolate human bone-Gla protein from an EDTA extract of human cortical bone. Thus, this method offers the possibility of developing a complete library of monoclonal antibodies against these and other bone-specific proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Anderson N. G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P. D., Stenner D., Wahner H. W., Mann K. G., Riggs B. L. Increase in serum bone gamma-carboxyglutamic acid protein with aging in women. Implications for the mechanism of age-related bone loss. J Clin Invest. 1983 May;71(5):1316–1321. doi: 10.1172/JCI110882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Foster W. B., Katzmann J. A., Miller R. S., Nesheim M. E., Mann K. G. Monoclonal antibodies selective for the functional states of bovine factor V and factor Va. Thromb Res. 1982 Dec 1;28(5):649–661. doi: 10.1016/0049-3848(82)90156-6. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V., Lian J. B., Gallop P. M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann J. A., Nesheim M. E., Hibbard L. S., Mann K. G. Isolation of functional human coagulation factor V by using a hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Jan;78(1):162–166. doi: 10.1073/pnas.78.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Poser J. W., Esch F. S., Ling N. C., Price P. A. Isolation and sequence of the vitamin K-dependent protein from human bone. Undercarboxylation of the first glutamic acid residue. J Biol Chem. 1980 Sep 25;255(18):8685–8691. [PubMed] [Google Scholar]
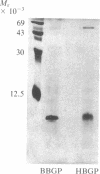
- Price P. A., Otsuka A. A., Poser J. W., Kristaponis J., Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976 May;73(5):1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Poser J. W., Raman N. Primary structure of the gamma-carboxyglutamic acid-containing protein from bovine bone. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3374–3375. doi: 10.1073/pnas.73.10.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasheva B., Dessev G. Artifacts in sodium dodecyl sulfate-polyacrylamide gel electrophoresis due to 2-mercaptoethanol. Anal Biochem. 1983 Feb 15;129(1):98–102. doi: 10.1016/0003-2697(83)90057-x. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Tracy R. P., Currie R. M., Young D. S. Two-dimensional gel electrophoresis of serum specimens from a normal population. Clin Chem. 1982 Apr;28(4 Pt 2):890–899. [PubMed] [Google Scholar]
- Triffitt J. T., Owen M. Preliminary studies on the binding of plasma albumin to bone tissue. Calcif Tissue Res. 1977 Oct 20;23(3):303–305. doi: 10.1007/BF02012801. [DOI] [PubMed] [Google Scholar]