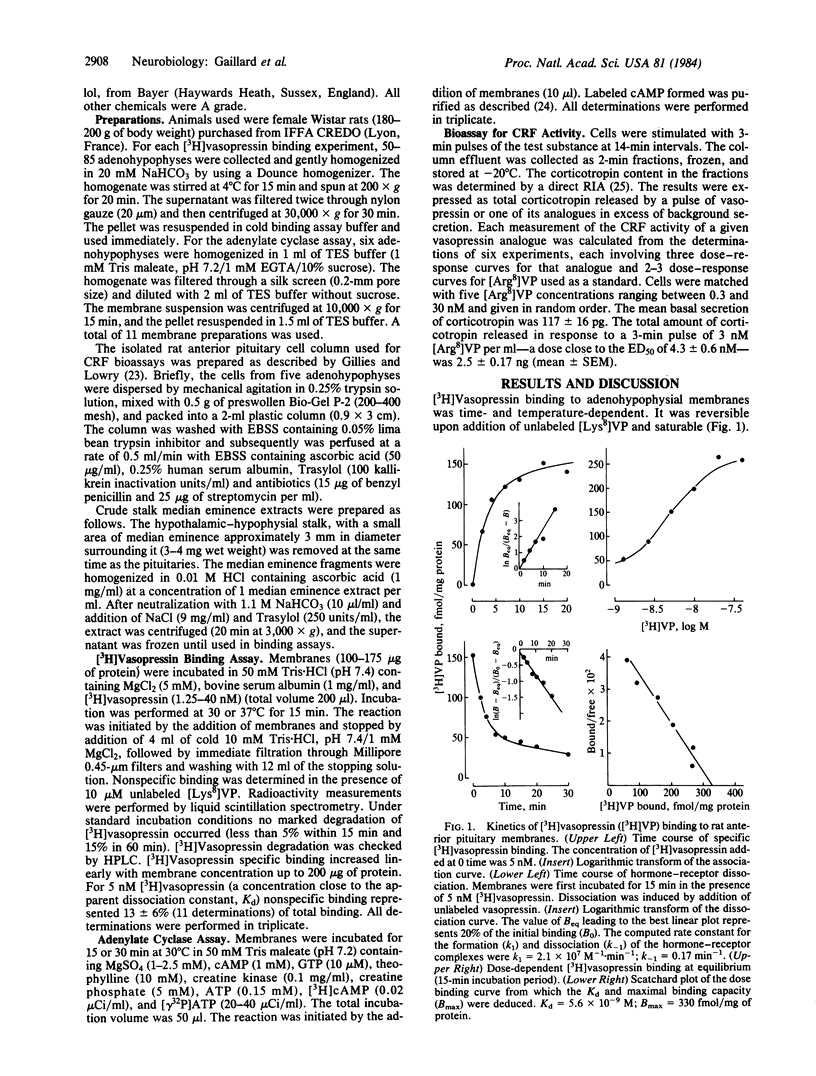
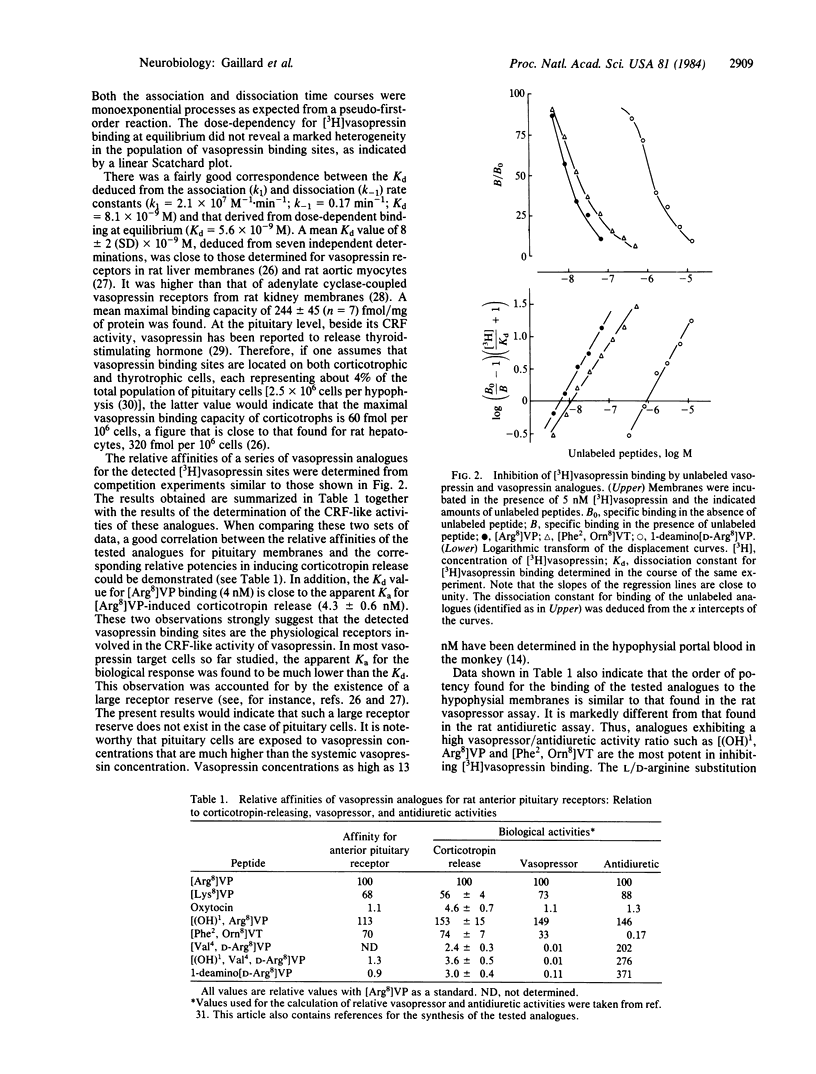
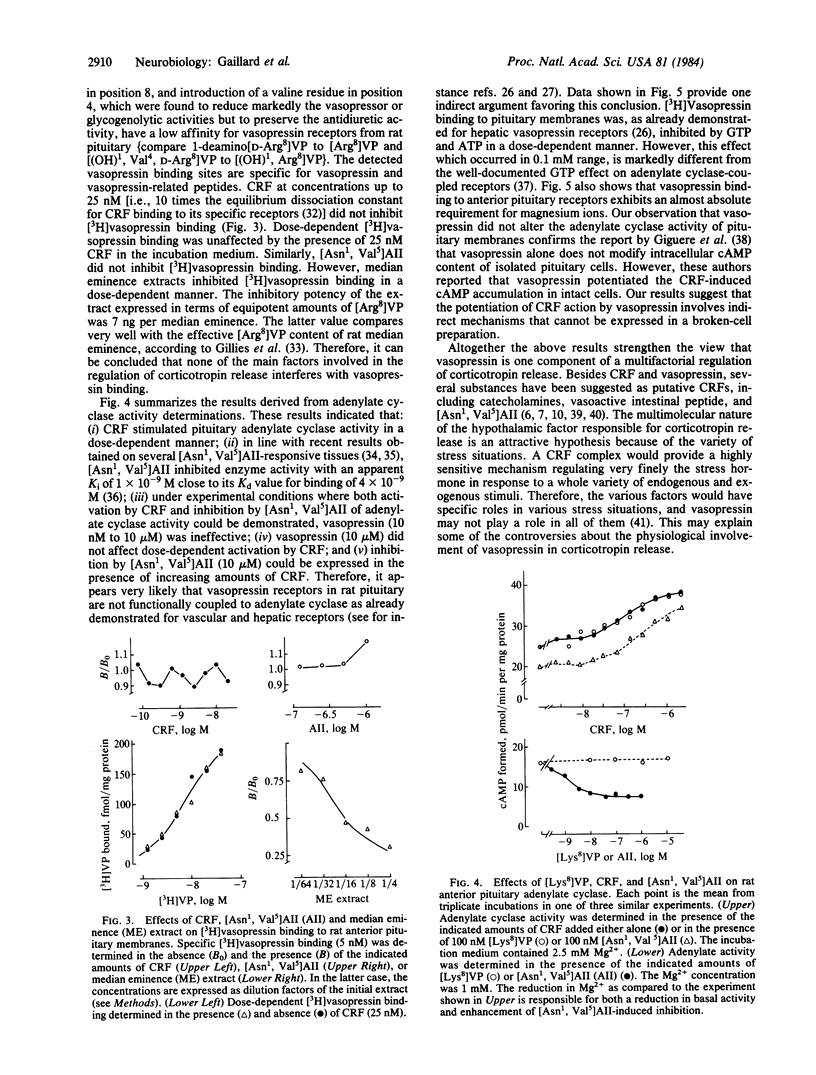
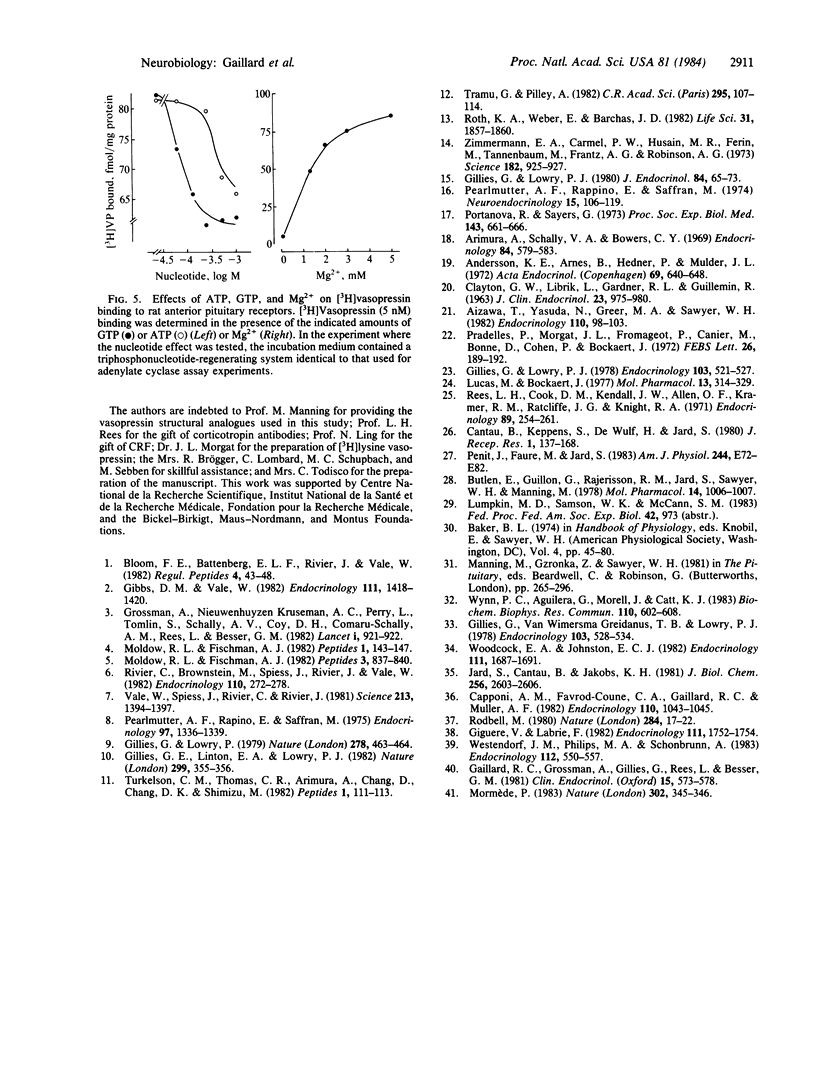
Abstract
Crude plasma membrane fractions were prepared from female Wistar rat anterior pituitaries. These fractions contained a single population of specific 3H-labeled [8-lysine]vasopressin [( 3H]vasopressin) binding sites with a dissociation of constant (Kd) of 8 +/- 2 X 10(-9) M and maximal binding capacity of 244 +/- 45 fmol/mg of protein. The Kd values for a series of vasopressin structural analogues with selective vasopressor or antidiuretic activities were determined together with the corresponding corticotropin-releasing activities (isolated perfused pituitary cells were used). A good correspondence was found between the two sets of values, suggesting that the detected vasopressin binding sites are the receptors involved in vasopressin-induced corticotropin release. The order of potency of these analogues for the binding to hypophysial receptors was similar to that found for the binding to the receptors involved in the vasopressor response. Corticotropin-releasing factor and angiotensin did not affect vasopressin binding to pituitary membranes. Median eminence extracts inhibited [3H]vasopressin binding with an efficiency very close to that expected from their vasopressin content. Corticotropin-releasing factor activated, and angiotensin inhibited, the adenylate cyclase activity of pituitary membranes. Under the same experimental conditions, vasopressin did not influence adenylate cyclase activity nor did it affect the corticotropin-releasing factor-induced activation. These data support the view that vasopressin is one component of the multifactorial regulation of corticotropin release and that it acts through a cAMP-independent pathway. The potentiation by vasopressin of corticotropin-releasing factor-induced cAMP accumulation in intact cells very likely proceeds through indirect mechanisms, which are not expressed in broken cell preparations.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa T., Yasuda N., Greer M. A., Sawyer W. H. In vivo adrenocorticotropin-releasing activity of neurohypophyseal hormones and their analogs. Endocrinology. 1982 Jan;110(1):98–104. doi: 10.1210/endo-110-1-98. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Arner B., Hedner P., Mulder J. L. Effects of 8-lysine-vasopressin and synthetic analogues of release of ACTH. Acta Endocrinol (Copenh) 1972 Apr;69(4):640–648. doi: 10.1530/acta.0.0690640. [DOI] [PubMed] [Google Scholar]
- Arimura A., Schally A. V., Bowers C. Y. Corticotropin releasing activity of lysine vasopressin analogues. Endocrinology. 1969 Mar;84(3):579–583. doi: 10.1210/endo-84-3-579. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Battenberg E. L., Rivier J., Vale W. Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul Pept. 1982 Jun;4(1):43–48. doi: 10.1016/0167-0115(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Butlen D., Guillon G., Rajerison R. M., Jard S., Sawyer W. H., Manning M. Structural requirements for activation of vasopressin-sensitive adenylate cyclase, hormone binding, and antidiuretic actions: effects of highly potent analogues and competitive inhibitors. Mol Pharmacol. 1978 Nov;14(6):1006–1017. [PubMed] [Google Scholar]
- CLAYTON G. W., LIBRIK L., GARDNER R. L., GUILLEMIN R. STUDIES ON THE CIRCADIAN RHYTHM OF PITUITARY ADRENOCORTICOTROPIC RELEASE IN MAN. J Clin Endocrinol Metab. 1963 Oct;23:975–980. doi: 10.1210/jcem-23-10-975. [DOI] [PubMed] [Google Scholar]
- Cantau B., Keppens S., De Wulf H., Jard S. (3H)-vasopressin binding to isolated rat hepatocytes and liver membranes: regulation by GTP and relation to glycogen phosphorylase activation. J Recept Res. 1980;1(2):137–168. doi: 10.3109/10799898009044096. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Favrod-Coune C. A., Gaillard R. C., Muller A. F. Binding and activation properties of angiotensin II in dispersed rat anterior pituitary cells. Endocrinology. 1982 Mar;110(3):1043–1045. doi: 10.1210/endo-110-3-1043. [DOI] [PubMed] [Google Scholar]
- Gaillard R. C., Grossman A., Gillies G., Rees L. H., Besser G. M. Angiotensin II stimulates the release of ACTH from dispersed rat anterior pituitary cells. Clin Endocrinol (Oxf) 1981 Dec;15(6):573–578. doi: 10.1111/j.1365-2265.1981.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Gibbs D. M., Vale W. Presence of corticotropin releasing factor-like immunoreactivity in hypophysial portal blood. Endocrinology. 1982 Oct;111(4):1418–1420. doi: 10.1210/endo-111-4-1418. [DOI] [PubMed] [Google Scholar]
- Giguere V., Labrie F. Vasopressin potentiates cyclic AMP accumulation and ACTH release induced by corticotropin-releasing factor (CRF) in rat anterior pituitary cells in culture. Endocrinology. 1982 Nov;111(5):1752–1754. doi: 10.1210/endo-111-5-1752. [DOI] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Gillies G., Lowry P. J. Corticotrophin releasing activity in extracts of the stalk median eminence of Brattleboro rats. J Endocrinol. 1980 Jan;84(1):65–73. doi: 10.1677/joe.0.0840065. [DOI] [PubMed] [Google Scholar]
- Gillies G., Lowry P. J. Perfused rat isolated anterior pituitary cell column as bioassay for factor(s) controlling release of adrenocorticotropin: validation of a technique. Endocrinology. 1978 Aug;103(2):521–527. doi: 10.1210/endo-103-2-521. [DOI] [PubMed] [Google Scholar]
- Gillies G., Lowry P. Corticotrophin releasing factor may be modulated vasopressin. Nature. 1979 Mar 29;278(5703):463–464. doi: 10.1038/278463a0. [DOI] [PubMed] [Google Scholar]
- Gillies G., Van Wimersma Greidanus T. B., Lowry P. J. Characterization of rat stalk median eminence vasopressin and its involvement in adrenocorticotropin release. Endocrinology. 1978 Aug;103(2):528–534. doi: 10.1210/endo-103-2-528. [DOI] [PubMed] [Google Scholar]
- Grossman A., Kruseman A. C., Perry L., Tomlin S., Schally A. V., Coy D. H., Rees L. H., Comaru-Schally A. M., Besser G. M. New hypothalamic hormone, corticotropin-releasing factor, specifically stimulates the release of adrenocorticotropic hormone and cortisol in man. Lancet. 1982 Apr 24;1(8278):921–922. doi: 10.1016/s0140-6736(82)91929-8. [DOI] [PubMed] [Google Scholar]
- Jard S., Cantau B., Jakobs K. H. Angiotensin II and alpha-adrenergic agonists inhibit rat liver adenylate cyclase. J Biol Chem. 1981 Mar 25;256(6):2603–2606. [PubMed] [Google Scholar]
- Lucas M., Bockaert J. Use of (-)-[3H]dihydroalprenolol to study beta adrenergic receptor-adenylate cyclase coupling in C6 glioma cells: role of 5'-guanylylimidodiphosphate. Mol Pharmacol. 1977 Mar;13(2):314–329. [PubMed] [Google Scholar]
- Moldow R. L., Fischman A. J. Hypothalamic CRF-like immunoreactivity in the rat after hypophysectomy or adrenalectomy. Peptides. 1982 Mar-Apr;3(2):143–147. doi: 10.1016/0196-9781(82)90043-2. [DOI] [PubMed] [Google Scholar]
- Moldow R. L., Fischman A. J. Physiological changes in rat hypothalamic CRF: circadian, stress and steroid suppression. Peptides. 1982 Sep-Oct;3(5):837–840. doi: 10.1016/0196-9781(82)90024-9. [DOI] [PubMed] [Google Scholar]
- Mormède P. The vasopressin receptor antagonist dPTyr (Me) AVP does not prevent stress-induced ACTH and corticosterone release. Nature. 1983 Mar 24;302(5906):345–346. doi: 10.1038/302345a0. [DOI] [PubMed] [Google Scholar]
- Pearlmutter A. F., Rapino E., Saffran M. A semi-automated in vitro assay for CRF: activities of peptides related to oxytocin and vasopressin. Neuroendocrinology. 1974;15(2):106–119. doi: 10.1159/000122299. [DOI] [PubMed] [Google Scholar]
- Pearlmutter A. F., Rapino E., Saffran M. The ACTH-releasing hormone of the hypothalamus requires a co-factor. Endocrinology. 1975 Nov;97(5):1336–1339. doi: 10.1210/endo-97-5-1336. [DOI] [PubMed] [Google Scholar]
- Penit J., Faure M., Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol. 1983 Jan;244(1):E72–E82. doi: 10.1152/ajpendo.1983.244.1.E72. [DOI] [PubMed] [Google Scholar]
- Portanova R., Sayers G. Isolated pituitary cells: CRF-like activity of neurohypophysial and related polypeptides. Proc Soc Exp Biol Med. 1973 Jul;143(3):661–666. doi: 10.3181/00379727-143-37386. [DOI] [PubMed] [Google Scholar]
- Pradelles P., Morgat J. L., Fromageot P., Camier M., Bonne D., Cohen P., Bockaert J., Jard S. Tritium labelling of 8-lysine vasopressin and its purification by affinity chromatography on sepharose bound neurophysins. FEBS Lett. 1972 Oct 1;26(1):189–192. doi: 10.1016/0014-5793(72)80570-2. [DOI] [PubMed] [Google Scholar]
- Rees L. H., Cook D. M., Kendall J. W., Allen C. F., Kramer R. M., Ratcliffe J. G., Knight R. A. A radioimmunoassay for rat plasma ACTH. Endocrinology. 1971 Jul;89(1):254–261. doi: 10.1210/endo-89-1-254. [DOI] [PubMed] [Google Scholar]
- Rivier C., Brownstein M., Spiess J., Rivier J., Vale W. In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin, beta-endorphin, and corticosterone. Endocrinology. 1982 Jan;110(1):272–278. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Weber E., Barchas J. D. Immunoreactive corticotropin releasing factor (CRF) and vasopressin are colocalized in a subpopulation of the immunoreactive vasopressin cells in the paraventricular nucleus of the hypothalamus. Life Sci. 1982 Oct 18;31(16-17):1857–1860. doi: 10.1016/0024-3205(82)90228-4. [DOI] [PubMed] [Google Scholar]
- Tramu G., Pillez A. Localisation immunohistochimique des terminaisons nerveuses à corticolibérine (GRF) dans I'éminence médiane du Cobaye et du Rat. C R Seances Acad Sci III. 1982 Jan 11;294(2):107–114. [PubMed] [Google Scholar]
- Turkelson C. M., Thomas C. R., Arimura A., Chang D., Chang J. K., Shimizu M. In vitro potentiation of the activity of synthetic ovine corticotropin-releasing factor by arginine vasopressin. Peptides. 1982 Mar-Apr;3(2):111–113. doi: 10.1016/0196-9781(82)90037-7. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Phillips M. A., Schonbrunn A. Vasoactive intestinal peptide stimulates hormone release from corticotropic cells in culture. Endocrinology. 1983 Feb;112(2):550–557. doi: 10.1210/endo-112-2-550. [DOI] [PubMed] [Google Scholar]
- Woodcock E. A., Johnston C. I. Inhibition of adenylate cyclase by angiotensin II in rat renal cortex. Endocrinology. 1982 Nov;111(5):1687–1691. doi: 10.1210/endo-111-5-1687. [DOI] [PubMed] [Google Scholar]
- Wynn P. C., Aguilera G., Morell J., Catt K. J. Properties and regulation of high-affinity pituitary receptors for corticotropin-releasing factor. Biochem Biophys Res Commun. 1983 Jan 27;110(2):602–608. doi: 10.1016/0006-291x(83)91192-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman E. A., Carmel P. W., Husain M. K., Ferin M., Tannenbaum M., Frantz A. G., Robinson A. G. Vasopressin and neurophysin: high concentrations in monkey hypophyseal portal blood. Science. 1973 Nov 20;182(4115):925–927. doi: 10.1126/science.182.4115.925. [DOI] [PubMed] [Google Scholar]


