Abstract
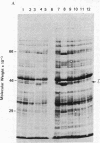
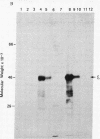
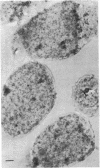
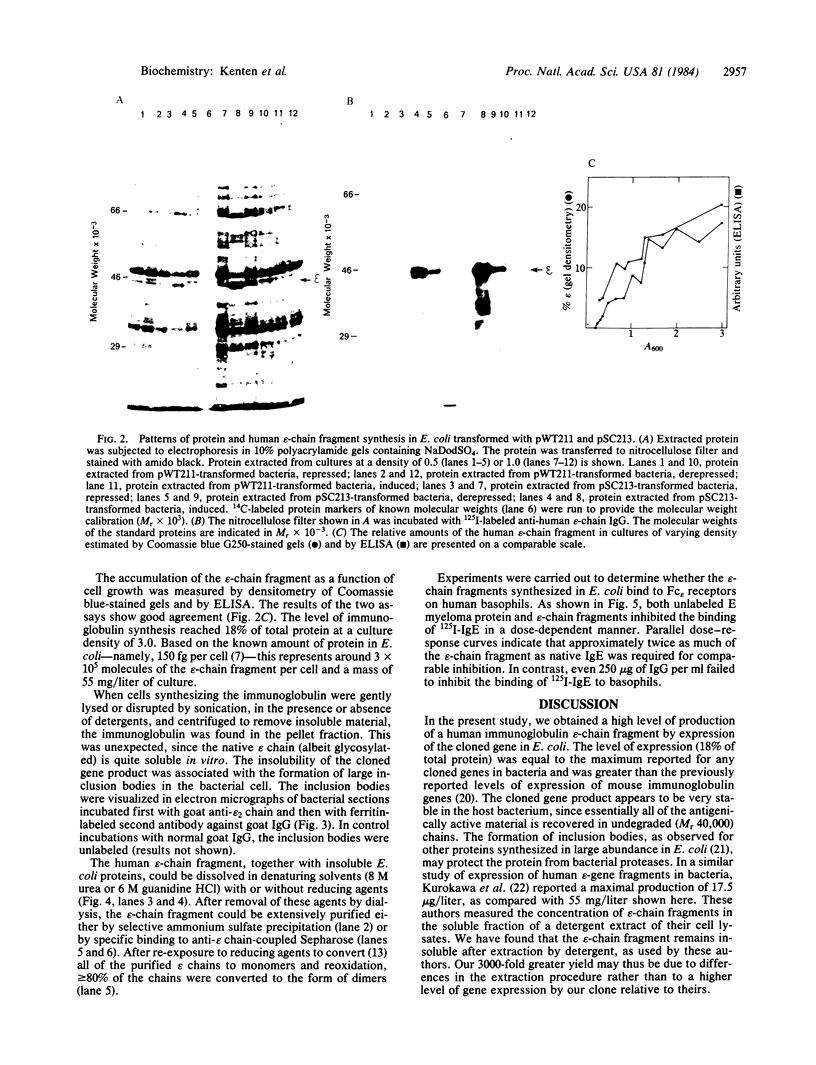
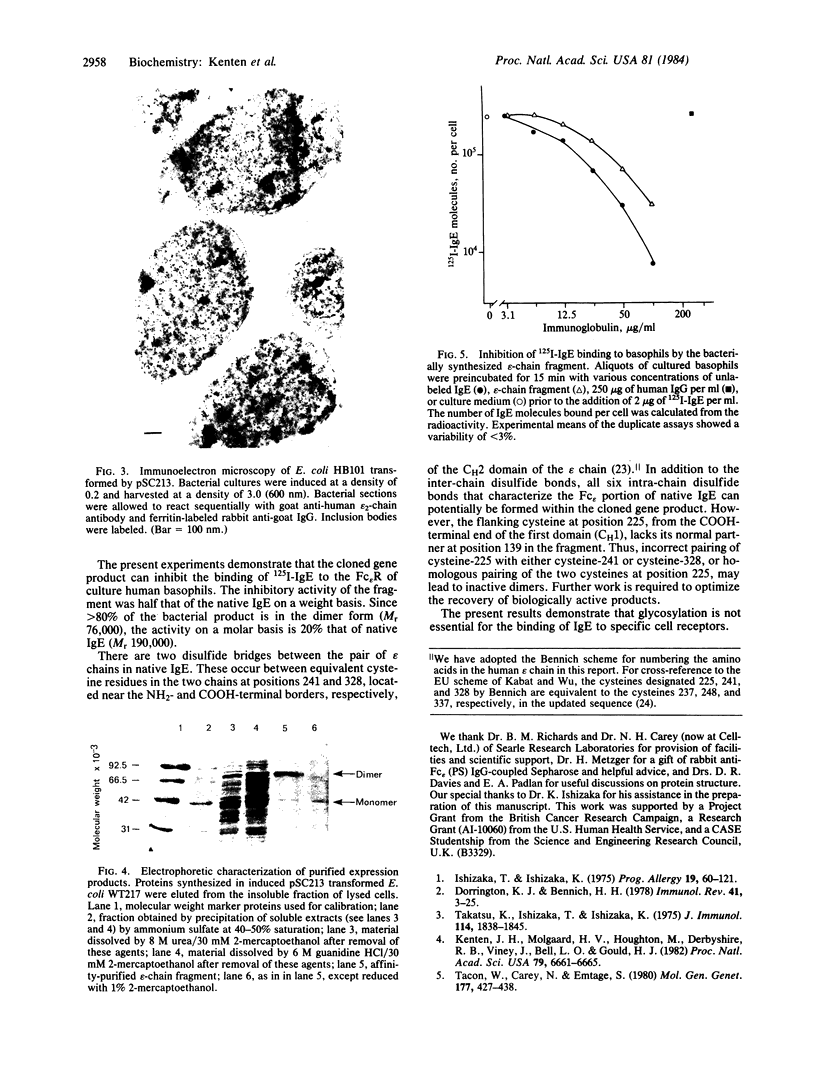
A fragment of the cloned gene for the human myeloma ND epsilon chain, coding for the second, third, and fourth domains of the immunoglobulin, has been coupled to the tryptophan control region of an expression plasmid and subcloned in Escherichia coli. Induction of gene expression results in the synthesis of the expected, antigenically active polypeptide of Mr 40,000, which constitutes 18% of total bacterial protein and yields 55 mg/liter of culture. The immunoglobulin, which is aggregated and packed into large inclusion bodies within the bacterial cell, can be dissolved by denaturing solvents and purified by affinity chromatography using anti-IgE Sepharose. Reduced monomeric chains assemble spontaneously into dimers. On assay to measure the inhibition of binding of 125I-labeled human E myeloma protein to Fc epsilon receptors on cultured human basophils, the cloned gene product exhibited 20% of the activity of the native protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dorrington K. J., Bennich H. H. Structure-function relationships in human immunoglobulin E. Immunol Rev. 1978;41:3–25. doi: 10.1111/j.1600-065x.1978.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Emtage J. S., Angal S., Doel M. T., Harris T. J., Jenkins B., Lilley G., Lowe P. A. Synthesis of calf prochymosin (prorennin) in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3671–3675. doi: 10.1073/pnas.80.12.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Lee E. H. Biologic function of the Fc fragments of E myeloma protein. Immunochemistry. 1970 Aug;7(8):687–702. doi: 10.1016/0019-2791(70)90175-8. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Biology of immunoglobulin E. Molecular basis of reaginic hypersensitivity. Prog Allergy. 1975;19:60–121. [PubMed] [Google Scholar]
- Kenten J. H., Molgaard H. V., Houghton M., Derbyshire R. B., Viney J., Bell L. O., Gould H. J. Cloning and sequence determination of the gene for the human immunoglobulin epsilon chain expressed in a myeloma cell line. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6661–6665. doi: 10.1073/pnas.79.21.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Metzger H. The interaction of IgE with rat basophilic leukemia cells. II. Quantitative aspects of the binding reaction. J Exp Med. 1974 Dec 1;140(6):1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa T., Seno M., Sasada R., Ono Y., Onda H., Igarashi K., Kikuchi M., Sugino Y., Honjo T. Expression of human immunoglobulin E epsilon chain cDNA in E. coli. Nucleic Acids Res. 1983 May 25;11(10):3077–3085. doi: 10.1093/nar/11.10.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Nakahata T., Leary A. G., Sterk A. R., Ishizaka K., Ishizaka T. Suspension culture of human mast cells/basophils from umbilical cord blood mononuclear cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4494–4498. doi: 10.1073/pnas.80.14.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. G., Dorrington K. J. An in vitro system for studying the kinetics of interchain disulfide bond formation in immunoglobulin G. J Biol Chem. 1974 Sep 10;249(17):5633–5641. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tacon W., Carey N., Emtage S. The construction and characterisation of plasmid vectors suitable for the expression of all DNA phases under the control of the E. coli tryptophan promoter. Mol Gen Genet. 1980 Feb;177(3):427–438. doi: 10.1007/BF00271481. [DOI] [PubMed] [Google Scholar]
- Takatsu K., Ishizaka T., Ishizaka K. Biologic significance of disulfide bonds in human IgE molecules. J Immunol. 1975 Jun;114(6):1838–1845. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Van Frank R. M., Muth W. L., Burnett J. P. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982 Feb 5;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]