Abstract
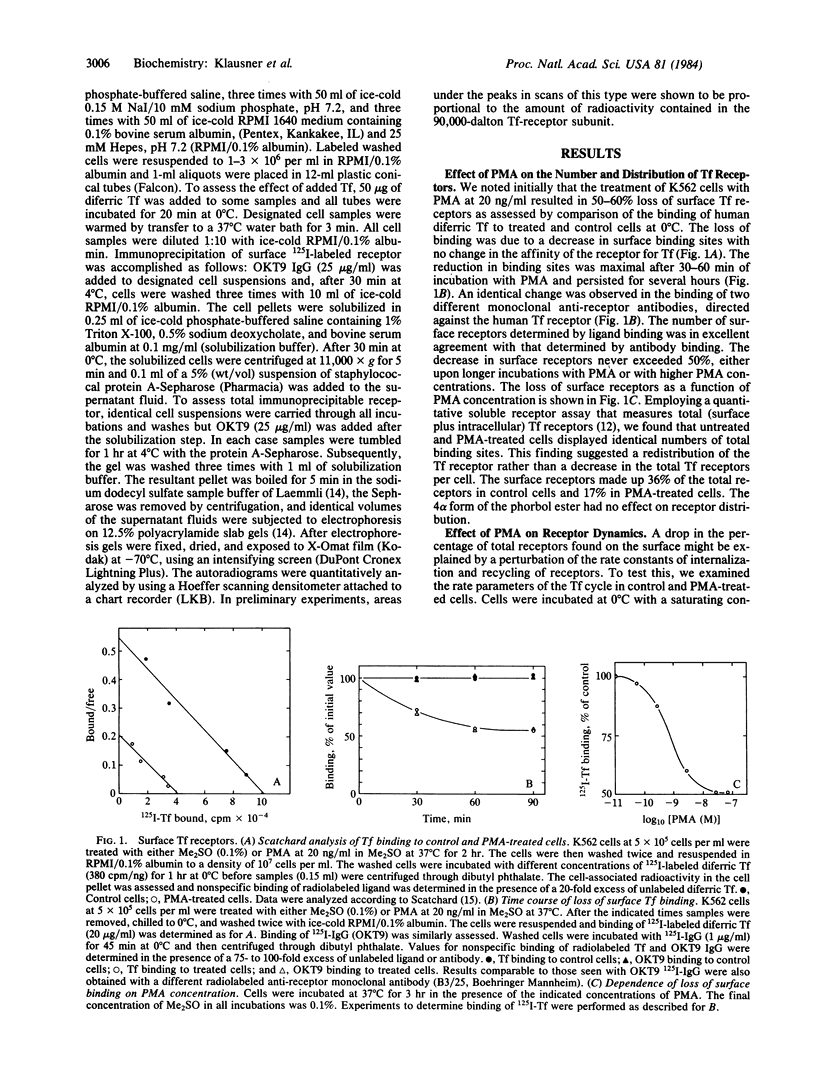
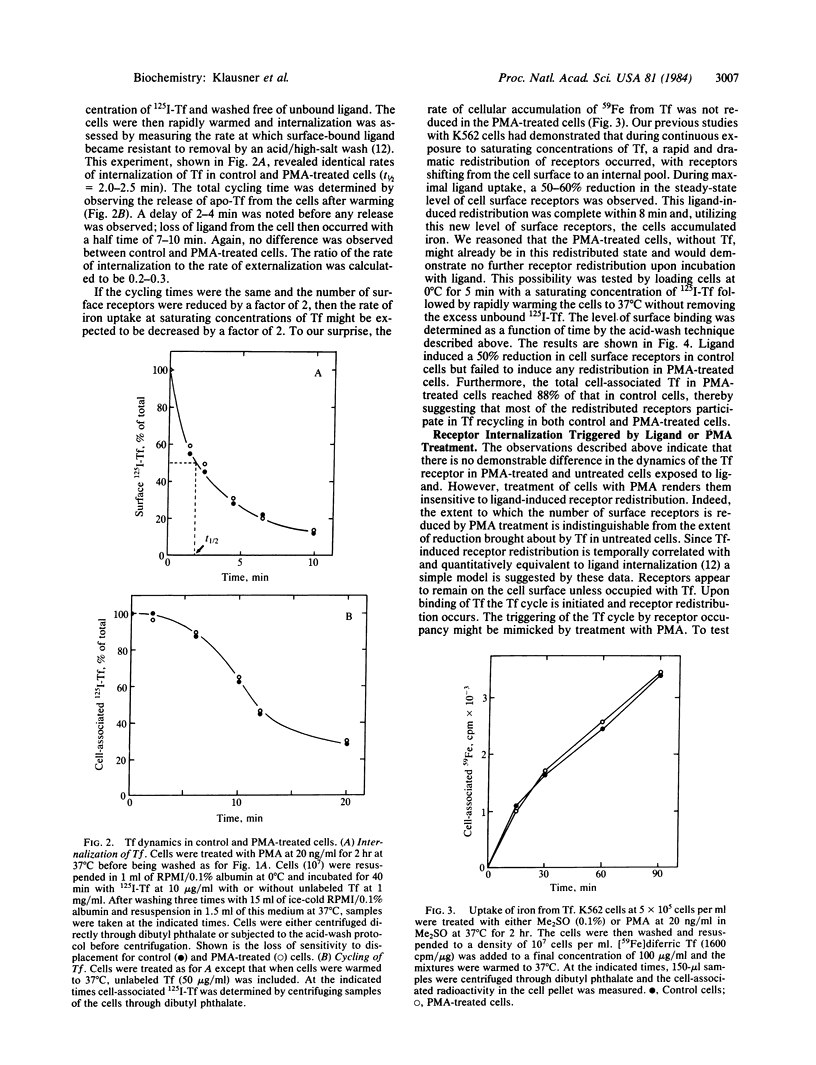
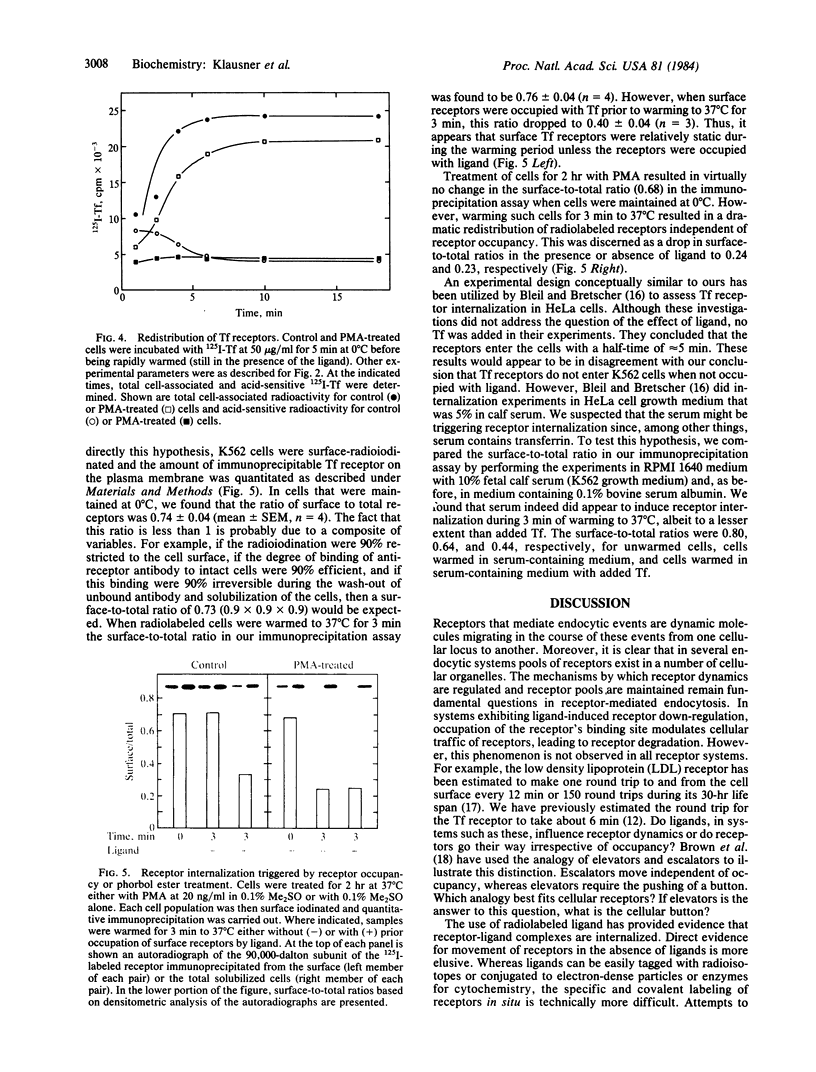
Treatment of human K562 cells with 4 beta-phorbol 12-myristate 13-acetate (PMA) resulted in an approximately 50% reduction in cell surface transferrin receptors within 30-45 min as judged by binding of both ligand and anti-receptor antibody. The affinity of the remaining surface receptors for diferric transferrin appeared to be unaltered. The time-dependent loss in transferrin receptors was also dependent upon PMA concentration, with a half-maximal effect observed at approximately 1 nM. The kinetic parameters for the binding, internalization, intracellular residency, and recycling of 125I-labeled transferrin were unchanged by PMA treatment, as were the rate and extent of internalization of anti-receptor antibody. Moreover, despite the decrease in surface receptors, uptake of 59Fe from transferrin proceeded at a rate comparable to that seen in untreated cells. Accounting for this observation was the fact that ligand induced a reduction in surface receptors in untreated but not PMA-treated cells. Quantitative immunoprecipitation of transferrin receptors from surface-iodinated K562 cells revealed that little receptor internalization occurred in untreated cells in the absence of ligand, but internalization of ligand-occupied receptors in these cells was readily detected. In contrast, PMA treatment resulted in the rapid internalization of surface receptors irrespective of occupancy. Thus, binding of ligand appeared to trigger the internalization of receptors that were relatively static in their unoccupied state, and a signal for receptor internalization was also provided by PMA treatment. The possibility that this signal involves phosphorylation of the transferrin receptor is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Berg T., Blomhoff R., Naess L., Tolleshaug H., Drevon C. A. Monensin inhibits receptor-mediated endocytosis of asialoglycoproteins in rat hepatocytes. Exp Cell Res. 1983 Oct 15;148(2):319–330. doi: 10.1016/0014-4827(83)90156-8. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Bretscher M. S. Transferrin receptor and its recycling in HeLa cells. EMBO J. 1982;1(3):351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Basu S. K., Goldstein J. L. Recycling of cell-surface receptors: observations from the LDL receptor system. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):713–721. doi: 10.1101/sqb.1982.046.01.068. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Lodish H. F. The asialoglycoprotein receptor internalizes and recycles independently of the transferrin and insulin receptors. Cell. 1983 Jan;32(1):267–275. doi: 10.1016/0092-8674(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. Specific binding of phorbol ester tumor promoters. Proc Natl Acad Sci U S A. 1980 Jan;77(1):567–571. doi: 10.1073/pnas.77.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns C. A., Larrick J. W., Suomalainen H., Schroder J., Sussman H. H. Co-migration and internalization of transferrin and its receptor on K562 cells. J Cell Biol. 1983 Aug;97(2):579–585. doi: 10.1083/jcb.97.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete D., Brownell M. D., Baenziger J. U. Evidence for transmembrane modulation of the ligand-binding site of the hepatocyte galactose/N-acetylgalactosamine-specific receptor. J Biol Chem. 1983 Jan 25;258(2):817–823. [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne R., Tashjian A. H., Jr Modulation of peptide binding to specific receptors on rat pituitary cells by tumor-promoting phorbol esters: decreased binding of thyrotropin-releasing hormone and somatostatin as well as epidermal growth factor. Cancer Res. 1982 Nov;42(11):4375–4381. [PubMed] [Google Scholar]
- Robbins A. R., Peng S. S., Marshall J. L. Mutant Chinese hamster ovary cells pleiotropically defective in receptor-mediated endocytosis. J Cell Biol. 1983 Apr;96(4):1064–1071. doi: 10.1083/jcb.96.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando J. J., Young M. C. Identification of high-affinity phorbol ester receptor in cytosol of EL4 thymoma cells: requirement for calcium, magnesium, and phospholipids. Proc Natl Acad Sci U S A. 1983 May;80(9):2642–2646. doi: 10.1073/pnas.80.9.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Chloroquine and ammonium ion inhibit receptor-mediated endocytosis of mannose-glycoconjugates by macrophages: apparent inhibition of receptor recycling. Biochem Biophys Res Commun. 1980 Mar 13;93(1):1–8. doi: 10.1016/s0006-291x(80)80237-3. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T. Chloroquine reduces the number of asialo-glycoprotein receptors in the hepatocyte plasma membrane. Biochem Pharmacol. 1979 Oct 1;28(19):2919–2922. doi: 10.1016/0006-2952(79)90586-0. [DOI] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Primary amines inhibit recycling of alpha 2M receptors in fibroblasts. Cell. 1980 May;20(1):37–43. doi: 10.1016/0092-8674(80)90232-9. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Lee L. S., Fisher P. B., Mufson A., Yamasaki H. Action of phorbol esters in cell culture: mimicry of transformation, altered differentiation, and effects on cell membranes. J Supramol Struct. 1979;12(2):195–208. doi: 10.1002/jss.400120206. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]




