Abstract
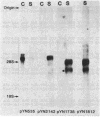
Hyaline cartilage contains a unique set of collagenous proteins. Type II collagen is the most abundant, constituting about 85% of the total cartilage collagen. In addition, several minor collagenous components have been described. To study the structure and developmental regulation of chondrocyte-specific collagens, we have constructed a cDNA library from embryonic chicken sternal cartilage mRNA. We report here on the isolation and characterization of a 3200 base-pair-long cDNA that codes for a collagenous polypeptide of unusual structure in that the total length of the molecule is only about half of pro alpha 1(II) collagen chains. The mRNA for this polypeptide is considerably smaller than mRNA encoding the pro alpha chains of interstitial collagens. In addition, the peptide encoded by the cDNA appears to contain at least three domains with triple-helical potential separated by short, noncollagenous peptides. Between the three collagenous domains are several cysteinyl residues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S., Abedin M. Z., Grundy S. M., Weiss J. B. Isolation and characterisation of an unusual collagen from hyaline cartilage and intervertebral disc. FEBS Lett. 1981 Jan 26;123(2):195–199. doi: 10.1016/0014-5793(81)80286-4. [DOI] [PubMed] [Google Scholar]
- Ayad S., Abedin M. Z., Weiss J. B., Grundy S. M. Characterisation of another short-chain disulphide-bonded collagen from cartilage, vitreous and intervertebral disc. FEBS Lett. 1982 Mar 22;139(2):300–304. doi: 10.1016/0014-5793(82)80875-2. [DOI] [PubMed] [Google Scholar]
- Bentz H., Morris N. P., Murray L. W., Sakai L. Y., Hollister D. W., Burgeson R. E. Isolation and partial characterization of a new human collagen with an extended triple-helical structural domain. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3168–3172. doi: 10.1073/pnas.80.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Mayne R., Tuderman L. p-HMW-collagen, a minor collagen obtained from chick embryo cartilage without proteolytic treatment of the tissue. Eur J Biochem. 1983 Nov 2;136(2):333–339. doi: 10.1111/j.1432-1033.1983.tb07746.x. [DOI] [PubMed] [Google Scholar]
- Burnett W., Rosenbloom J. Isolation and translation of elastin mRNA from chick aorta. Biochem Biophys Res Commun. 1979 Feb 14;86(3):478–484. doi: 10.1016/0006-291x(79)91739-x. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Wiedemann H., Timpl R., Odermatt E., Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983 May 1;211(2):303–311. doi: 10.1042/bj2110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Characterization of a unique collagenous fraction from limited pepsin digests of human placental tissue: molecular organization of the native aggregate. Biochemistry. 1981 Mar 17;20(6):1635–1640. doi: 10.1021/bi00509a035. [DOI] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Isolation of a unique collagenous fraction from limited pepsin digests of human placental tissue. Characterization of one of the constituent polypeptide chains. J Biol Chem. 1980 Jan 10;255(1):290–295. [PubMed] [Google Scholar]
- Gibson G. J., Kielty C. M., Garner C., Schor S. L., Grant M. E. Identification and partial characterization of three low-molecular-weight collagenous polypeptides synthesized by chondrocytes cultured within collagen gels in the absence and in the presence of fibronectin. Biochem J. 1983 May 1;211(2):417–426. doi: 10.1042/bj2110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Schor S. L., Grant M. E. Effects of matrix macromolecules on chondrocyte gene expression: synthesis of a low molecular weight collagen species by cells cultured within collagen gels. J Cell Biol. 1982 Jun;93(3):767–774. doi: 10.1083/jcb.93.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Glanville R. W. Further characterization of the three polypeptide chains of bovine and human short-chain collagen (intima collagen). Eur J Biochem. 1983 Jun 1;133(1):39–46. doi: 10.1111/j.1432-1033.1983.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Voss B., von Bassewitz D. B. A cysteine-rich collagenous protein from bovine placenta. Isolation of its constituent polypeptide chains and some properties of the non-denatured protein. Eur J Biochem. 1981;114(1):17–25. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Gay S. Collagen: an overview. Methods Enzymol. 1982;82(Pt A):3–32. doi: 10.1016/0076-6879(82)82058-2. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- Odermatt E., Risteli J., van Delden V., Timpl R. Structural diversity and domain composition of a unique collagenous fragment (intima collagen) obtained from human placenta. Biochem J. 1983 May 1;211(2):295–302. doi: 10.1042/bj2110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICH A., CRICK F. H. The molecular structure of collagen. J Mol Biol. 1961 Oct;3:483–506. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Wiedemann H., Kühn K., Mayne R. Characterization of a highly soluble collagenous molecule isolated from chicken hyaline cartilage. Biochemistry. 1982 Mar 2;21(5):826–830. doi: 10.1021/bi00534a002. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. A unique low molecular weight collagen secreted by cultured chick embryo chondrocytes. J Biol Chem. 1982 Oct 25;257(20):12444–12450. [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. A short chain (pro)collagen from aged endochondral chondrocytes. Biochemical characterization. J Biol Chem. 1983 Aug 10;258(15):9504–9509. [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of a new disulphide bonded collagen from cartilage. FEBS Lett. 1980 Nov 17;121(1):51–54. doi: 10.1016/0014-5793(80)81265-8. [DOI] [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of two further collagenous fractions from articular cartilage. Biosci Rep. 1981 Jul;1(7):561–570. doi: 10.1007/BF01116305. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K., van Menxel M., Wiedemann H. Isolation and characterization of a precursor form of M collagen from embryonic chicken cartilage. Eur J Biochem. 1984 Feb 1;138(3):629–633. doi: 10.1111/j.1432-1033.1984.tb07961.x. [DOI] [PubMed] [Google Scholar]
- von der Mark K., van Menxel M., Wiedemann H. Isolation and characterization of new collagens from chick cartilage. Eur J Biochem. 1982 May;124(1):57–62. doi: 10.1111/j.1432-1033.1982.tb05905.x. [DOI] [PubMed] [Google Scholar]