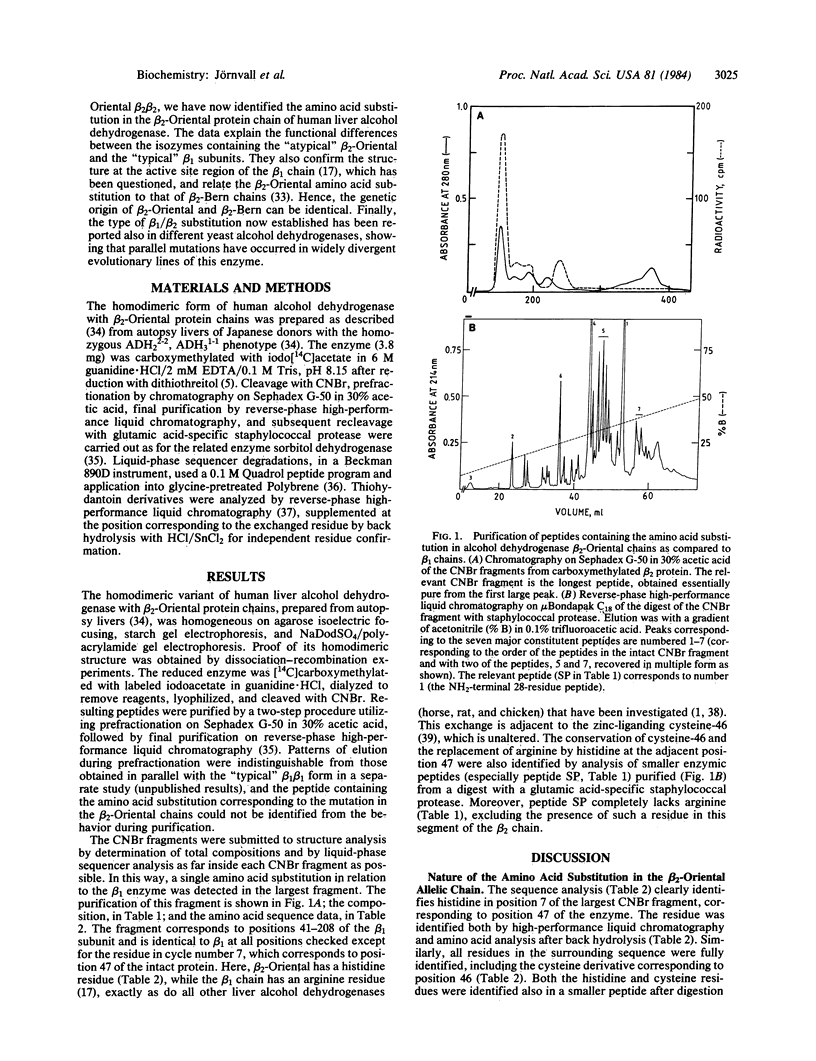
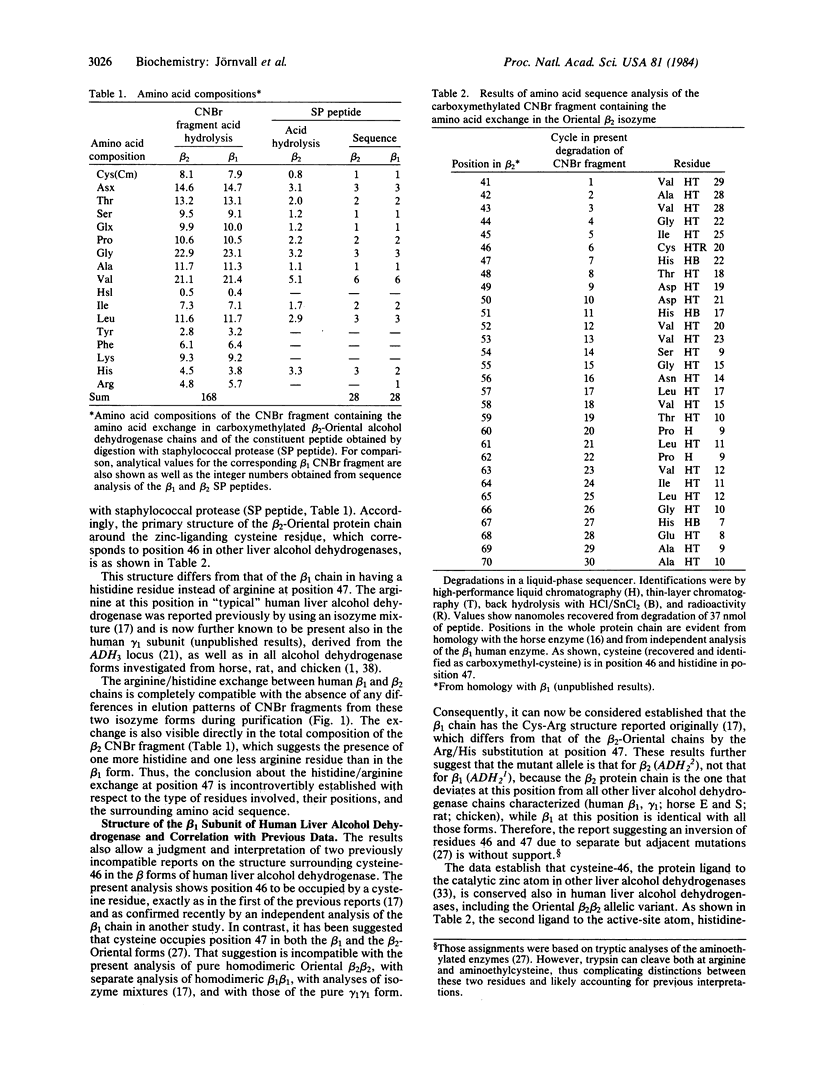
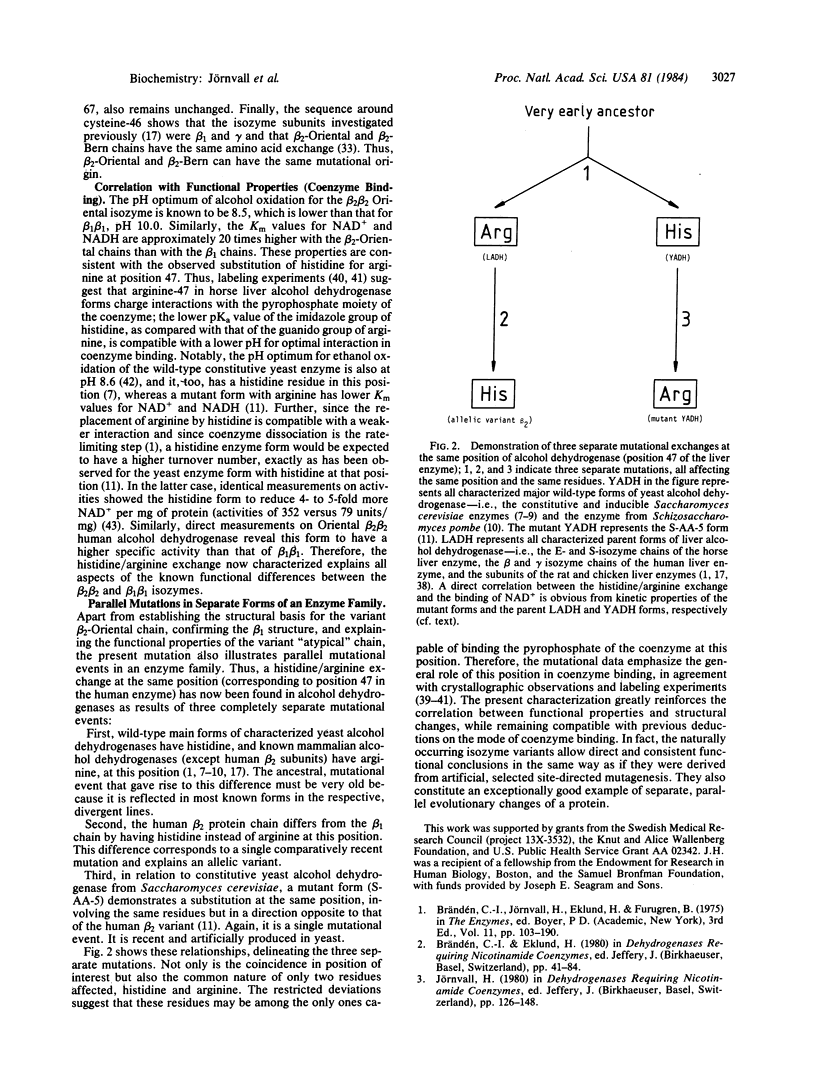
Abstract
The homodimeric Oriental beta 2 beta 2 isozyme of human liver alcohol dehydrogenase, corresponding to an allelic variant at the ADH2 gene locus, was studied in order to define the amino acid exchange in relation to the beta 1 beta 1 isozyme, the predominant allelic form among Caucasians. Sequence analysis reveals that the amino acid substitution occurs at position 7 of the largest CNBr fragment, corresponding to position 47 of the whole protein chain. Here, the beta 2 form has a histidine residue, while, in common with other characterized mammalian liver alcohol dehydrogenases, the beta 1 form has an arginine residue. This exchange does not affect the adjacent cysteine-46 residue, which is a protein ligand to the active-site zinc atom, thus clarifying previously inconsistent results. The histidine/arginine-47 mutational replacement corresponds to a position that binds the pyrophosphate group of the coenzyme NAD(H); this explains the functional differences between the beta 1 beta 1 and beta 2 beta 2 isozymes, including both a lower pH optimum and higher turnover number of beta 2 beta 2, which is likely to be the mutant form. The exchange demonstrates the existence of parallel but separate mutations in the evolution of alcohol dehydrogenases because these mammalian enzymes differ at exactly the same position by the same type of substitution as is found between a mutant and the wild-type constitutive forms of the corresponding yeast enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Benyajati C., Place A. R., Powers D. A., Sofer W. Alcohol dehydrogenase gene of Drosophila melanogaster: relationship of intervening sequences to functional domains in the protein. Proc Natl Acad Sci U S A. 1981 May;78(5):2717–2721. doi: 10.1073/pnas.78.5.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger D., Berger M., von Wartburg J. P. Structural studies of human-liver alcohol-dehydrogenase isoenzymes. Eur J Biochem. 1974 Dec 16;50(1):215–225. doi: 10.1111/j.1432-1033.1974.tb03890.x. [DOI] [PubMed] [Google Scholar]
- Bosron W. F., Li T. K. Genetic determinants of alcohol and aldehyde dehydrogenases and alcohol metabolism. Semin Liver Dis. 1981 Aug;1(3):179–188. doi: 10.1055/s-2008-1041746. [DOI] [PubMed] [Google Scholar]
- Bosron W. F., Li T. K., Vallee B. L. Heterogeneity and new molecular forms of human liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1549–1555. doi: 10.1016/0006-291x(79)91241-5. [DOI] [PubMed] [Google Scholar]
- Bosron W. F., Li T. K., Vallee B. L. New molecular forms of human liver alcohol dehydrogenase: isolation and characterization of ADHIndianapolis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5784–5788. doi: 10.1073/pnas.77.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosron W. F., Magnes L. J., Li T. K. Human liver alcohol dehydrogenase: ADH Indianapolis results from genetic polymorphism at the ADH2 gene locus. Biochem Genet. 1983 Aug;21(7-8):735–744. doi: 10.1007/BF00498920. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Eklund H., Samma J. P., Wallén L., Brändén C. I., Akeson A., Jones T. A. Structure of a triclinic ternary complex of horse liver alcohol dehydrogenase at 2.9 A resolution. J Mol Biol. 1981 Mar 15;146(4):561–587. doi: 10.1016/0022-2836(81)90047-4. [DOI] [PubMed] [Google Scholar]
- Fukui M., Wakasugi C. Liver alcohol dehydrogenase in a Japanese population. Nihon Hoigaku Zasshi. 1972 Jan;26(1):46–51. [PubMed] [Google Scholar]
- Jörnvall H., Eklund H., Brändén C. I. Subunit conformation of yeast alcohol dehydrogenase. J Biol Chem. 1978 Dec 10;253(23):8414–8419. [PubMed] [Google Scholar]
- Jörnvall H., Persson M., Jeffery J. Alcohol and polyol dehydrogenases are both divided into two protein types, and structural properties cross-relate the different enzyme activities within each type. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4226–4230. doi: 10.1073/pnas.78.7.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H., Philipson L. Limited proteolysis and a reactive cysteine residue define accesible regions in the native conformation of the adenovirus hexon protein. Eur J Biochem. 1980 Feb;104(1):237–247. doi: 10.1111/j.1432-1033.1980.tb04421.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Pietruszko R. Structural studies of alcohol dehydrogenase from human liver. Eur J Biochem. 1972 Feb 15;25(2):283–290. doi: 10.1111/j.1432-1033.1972.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. The primary structure of yeast alcohol dehydrogenase. Eur J Biochem. 1977 Feb;72(3):425–442. doi: 10.1111/j.1432-1033.1977.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Lange L. G., 3rd, Riordan J. F., Vallee B. L., Brändén C. I. The role of arginyl residues in directing carboxymethylation of horse liver alcohol dehydrogenase. Biochemistry. 1975 Jul 29;14(15):3497–3502. doi: 10.1021/bi00686a032. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Sytkowski A. J., Vallee B. L. Human liver alcohol dehydrogenase: purification, composition, and catalytic features. Biochemistry. 1976 Oct 19;15(21):4687–4693. doi: 10.1021/bi00666a023. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Vallee B. L. Double-ternary complex affinity chromatography: preparation of alcohol dehydrogenases. Biochemistry. 1976 Oct 19;15(21):4681–4686. doi: 10.1021/bi00666a022. [DOI] [PubMed] [Google Scholar]
- Li T. K., Bosron W. F., Dafeldecker W. P., Lange L. G., Vallee B. L. Isolation of pi-alcohol dehydrogenase of human liver: is it a determinant of alcoholism? Proc Natl Acad Sci U S A. 1977 Oct;74(10):4378–4381. doi: 10.1073/pnas.74.10.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parés X., Vallee B. L. New human liver alcohol dehydrogenase forms with unique kinetic characteristics. Biochem Biophys Res Commun. 1981 Jan 15;98(1):122–130. doi: 10.1016/0006-291x(81)91878-7. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. The primary structure of the alcohol dehydrogenase gene from the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1983 Jan 10;258(1):143–149. [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Developmental changes and polymorphism in human alcohol dehydrogenase. Ann Hum Genet. 1971 Feb;34(3):251–271. doi: 10.1111/j.1469-1809.1971.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Strydom D. J., Vallee B. L. Characterization of human alcohol dehydrogenase isoenzymes by high-performance liquid chromatographic peptide mapping. Anal Biochem. 1982 Jul 1;123(2):422–429. doi: 10.1016/0003-2697(82)90467-5. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R. The complete amino acid sequence of three alcohol dehydrogenase alleloenzymes (AdhN-11, AdhS and AdhUF) from the fruitfly Drosophila melanogaster. Biochem J. 1980 Jun 1;187(3):875–883. doi: 10.1042/bj1870875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLENFELS K., SUND H. Uber den Mechanismus der Wasserstoffübertragung mit Pyridinnucleotiden. I. Freie SH-Gruppen und Aktivität bei Alkoholdehydrogenase aus Hefe. Biochem Z. 1957;329(1):17–30. [PubMed] [Google Scholar]
- Wills C., Jörnvall H. Amino acid substitutions in two functional mutants of yeast alcohol dehydrogenase. Nature. 1979 Jun 21;279(5715):734–736. doi: 10.1038/279734a0. [DOI] [PubMed] [Google Scholar]
- Wills C., Jörnvall H. The two major isozymes of yeast alcohol dehydrogenase. Eur J Biochem. 1979 Sep;99(2):323–331. doi: 10.1111/j.1432-1033.1979.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Wills C. Production of yeast alcohol dehydrogenase isoenzymes by selection. Nature. 1976 May 6;261(5555):26–29. doi: 10.1038/261026a0. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Impraim C. C., Huang I. Y. Enzymatic and structural differences between usual and atypical human liver alcohol dehydrogenases. J Biol Chem. 1981 Dec 10;256(23):12430–12436. [PubMed] [Google Scholar]
- Zeppezauer E., Jörnvall H., Ohlsson I. Carboxymethylation of horse-liver alcohol dehydrogenase in the crystalline state. The active-site zinc region and general anion-binding site of the enzyme correlated in primary and teritiary structures. Eur J Biochem. 1975 Oct 1;58(1):95–104. doi: 10.1111/j.1432-1033.1975.tb02353.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]
- von Bahr-Lindström H., Andersson L., Mosbach K., Jörnvall H. Purification and characterization of chicken liver alcohol dehydrogenase. FEBS Lett. 1978 May 15;89(2):293–297. doi: 10.1016/0014-5793(78)80239-7. [DOI] [PubMed] [Google Scholar]
- von Wartburg J. P., Papenberg J., Aebi H. An atypical human alcohol dehydrogenase. Can J Biochem. 1965 Jul;43(7):889–898. doi: 10.1139/o65-102. [DOI] [PubMed] [Google Scholar]



