Abstract
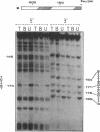
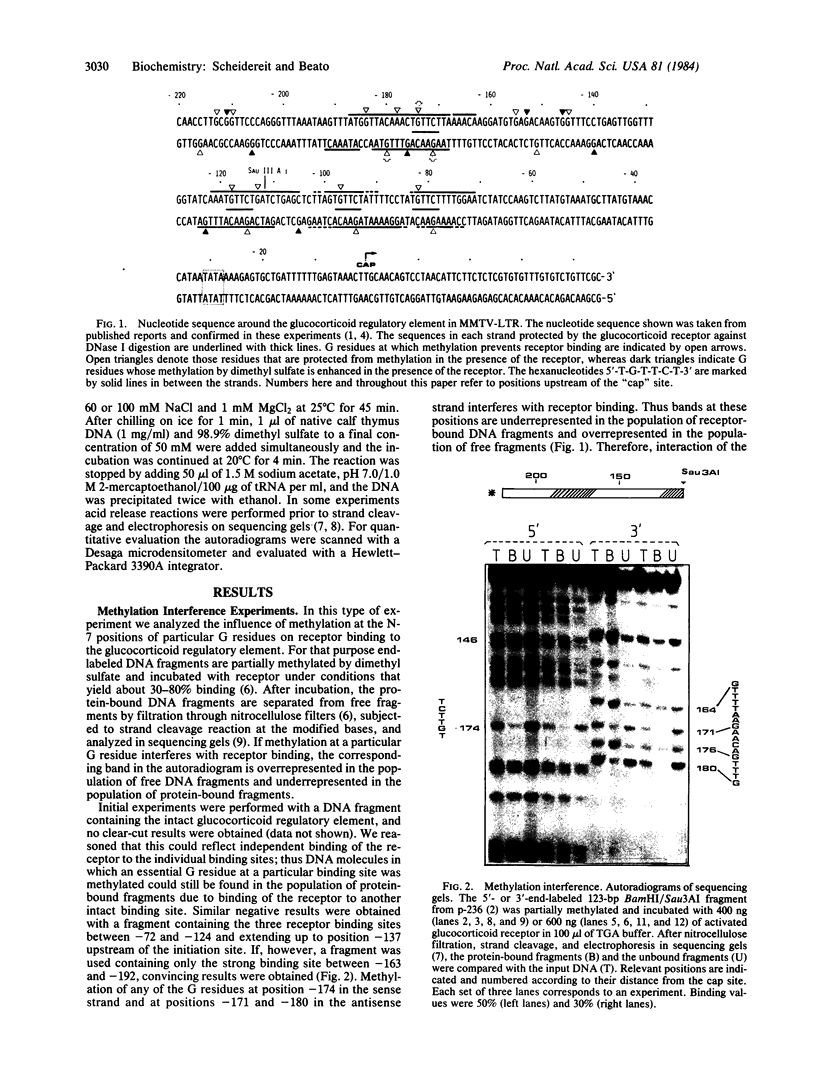
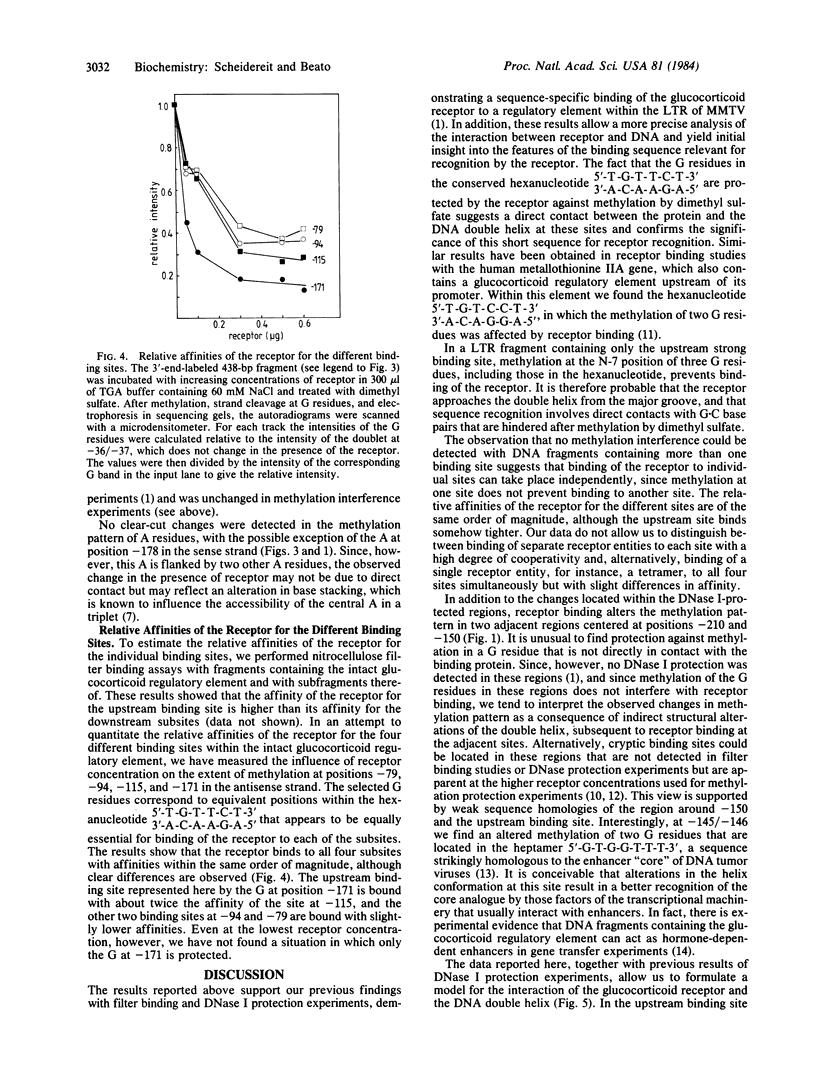
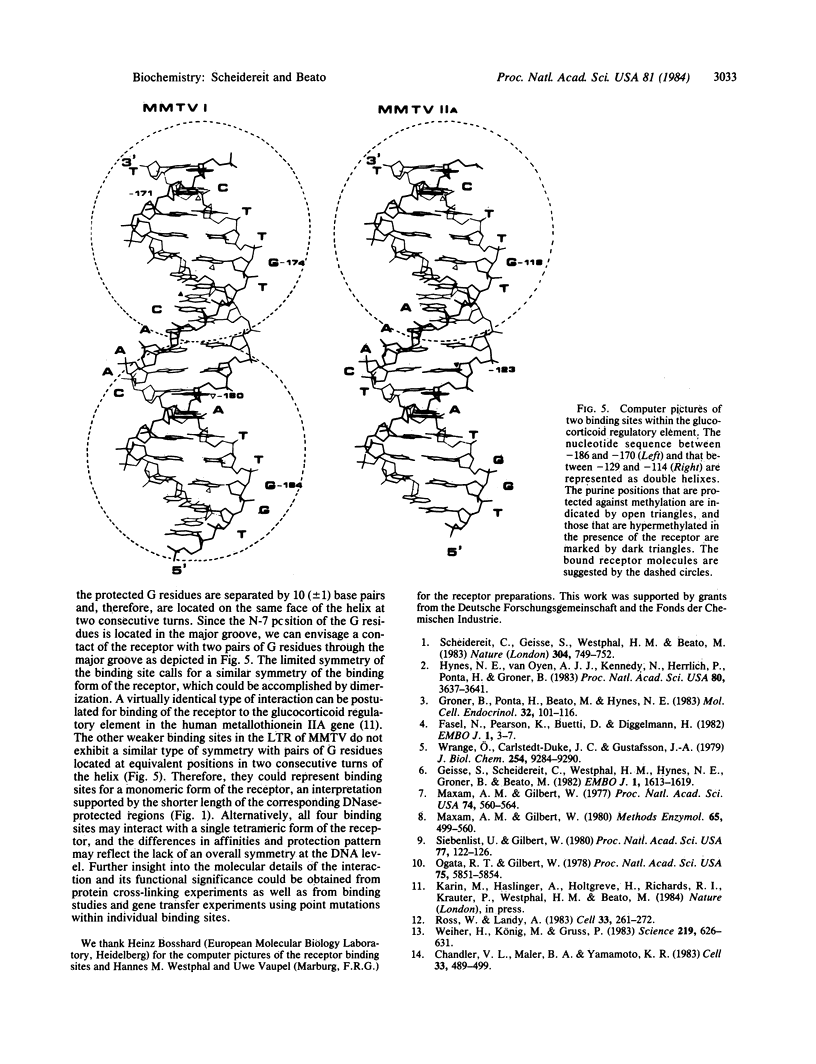
Glucocorticoid hormones enhance the transcription of mouse mammary tumor virus DNA by mechanisms involving a direct interaction of the hormone receptor with four binding sites in a glucocorticoid regulatory element located between -72 and -192 base pairs upstream of the main transcription initiation site within the proviral long terminal repeat regions. Methylation at the N-7 position of any of three G residues within one of the binding sites prevents binding of the receptor. In addition, in the presence of the receptor, methylation by dimethyl sulfate is reduced at several G residues, indicating sites of contact between the receptor and DNA at these positions. The G residues in the hexanucleotide 5'-T-G-T-T-C-T-3' 3'-A-C-A-A-G-A-5' were protected by the receptor against MH2-specific gene. (iii) myc is followed by the 3'-terminal c region of about 400 nucleotides, which is colinear with that of Rous sarcoma virus except for a substitution near the 5' end of the long terminal repeat. It is concluded that MH2 contains two genes with oncogenic potential, the delta gag- mht gene, which is closely related to the delta gag-raf transforming gene of MSV 3611, and the myc gene, which is related to the transforming gene of MC29. Furthermore, it may be concluded that the cellular proto-onc genes, which on sequence transduction become viral onc genes, are a small group because among the 19 known onc sequences, 5 are shared by different taxonomic groups of viruses of which the mht /raf homology is the closest determined so far.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Fasel N., Pearson K., Buetti E., Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1(1):3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisse S., Scheidereit C., Westphal H. M., Hynes N. E., Groner B., Beato M. Glucocorticoid receptors recognize DNA sequences in and around murine mammary tumour virus DNA. EMBO J. 1982;1(12):1613–1619. doi: 10.1002/j.1460-2075.1982.tb01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Ponta H., Beato M., Hynes N. E. The proviral DNA of mouse mammary tumor virus: its use in the study of the molecular details of steroid hormone action. Mol Cell Endocrinol. 1983 Oct;32(2-3):101–116. doi: 10.1016/0303-7207(83)90075-8. [DOI] [PubMed] [Google Scholar]
- Hynes N., van Ooyen A. J., Kennedy N., Herrlich P., Ponta H., Groner B. Subfragments of the large terminal repeat cause glucocorticoid-responsive expression of mouse mammary tumor virus and of an adjacent gene. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3637–3641. doi: 10.1073/pnas.80.12.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ogata R. T., Gilbert W. An amino-terminal fragment of lac repressor binds specifically to lac operator. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5851–5854. doi: 10.1073/pnas.75.12.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Landy A. Patterns of lambda Int recognition in the regions of strand exchange. Cell. 1983 May;33(1):261–272. doi: 10.1016/0092-8674(83)90355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wrange O., Carlstedt-Duke J., Gustafsson J. A. Purification of the glucocorticoid receptor from rat liver cytosol. J Biol Chem. 1979 Sep 25;254(18):9284–9290. [PubMed] [Google Scholar]