Abstract
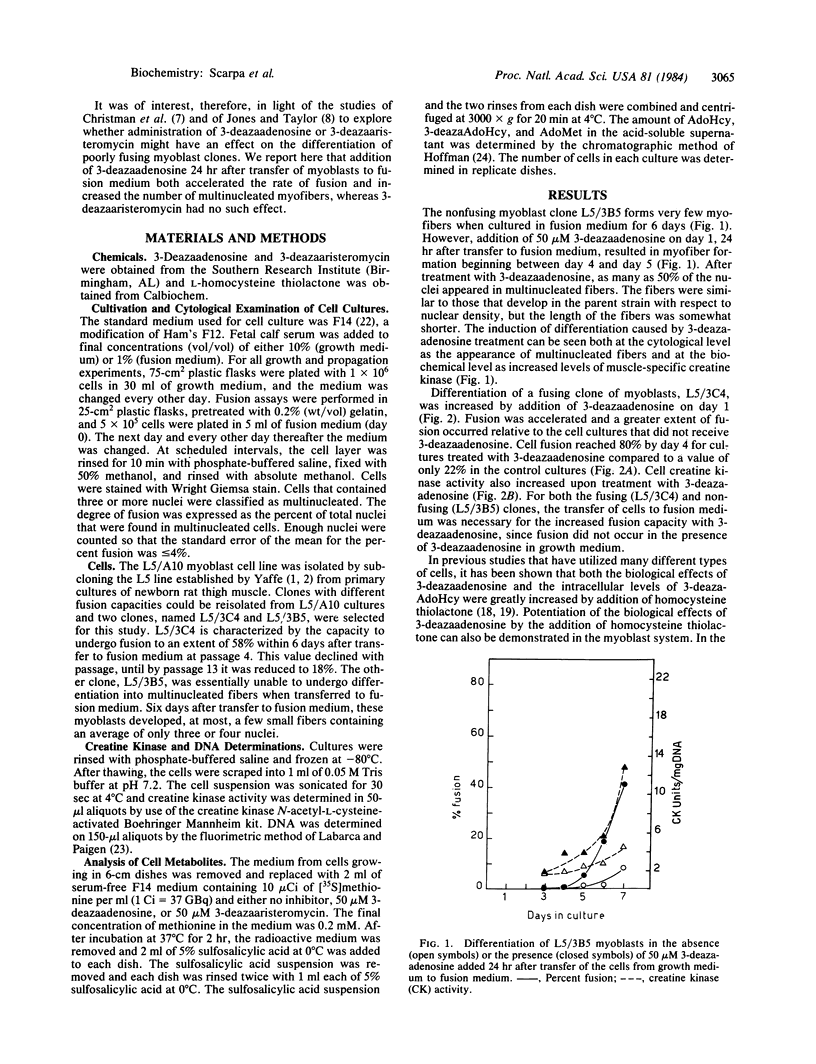
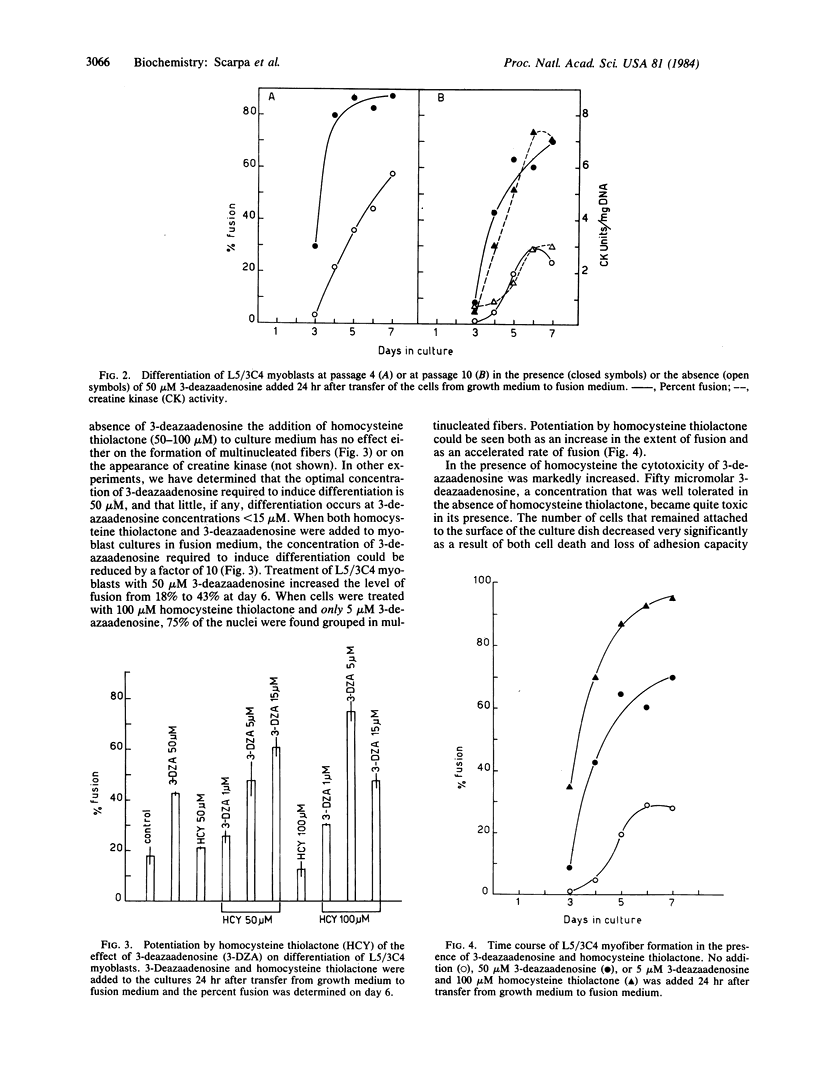
Treatment of myoblast cell lines with 3-deazaadenosine stimulates differentiation into myofibers. Myoblast clone L5/ 3B5 , which does not form myofibers after 6 days in fusion medium, was stimulated to form myofibers after 5 days of culture in fusion medium containing 50 microM 3-deazaadenosine. Myoblast clone L5/ 3C4 , which normally begins to form myofibers after 4 days in fusion medium, was stimulated by 50 microM 3-deazaadenosine to form myofibers after 3 days in culture and the extent of fusion was also increased. In the presence of 100 microM homocysteine thiolactone, the concentration of 3-deazaadenosine that stimulated maximal fusion was reduced by a factor of 10, from 50 microM to 5 microM 3-deazaadenosine. Stimulation of myofiber formation by 3-deazaadenosine suggests a requirement for one or more methylation reactions in myoblast differentiation and the potentiation by homocysteine thiolactone indicates that myofiber formation is specifically stimulated by an intracellular accumulation of 3-deazaadenosylhomocysteine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksamit R. R., Falk W., Cantoni G. L. Inhibition of chemotaxis by S-3-deazaadenosylhomocysteine in a mouse macrophage cell line. J Biol Chem. 1982 Jan 25;257(2):621–625. [PubMed] [Google Scholar]
- Bader J. P., Brown N. R., Chiang P. K., Cantoni G. L. 3-Deazaadenosine, an inhibitor of adenosylhomocysteine hydrolase, inhibits reproduction of Rous sarcoma virus and transformation of chick embryo cells. Virology. 1978 Sep;89(2):494–505. doi: 10.1016/0042-6822(78)90191-5. [DOI] [PubMed] [Google Scholar]
- Chiang P. K. Conversion of 3T3-L1 fibroblasts to fat cells by an inhibitor of methylation: effect of 3-deazaadenosine. Science. 1981 Mar 13;211(4487):1164–1166. doi: 10.1126/science.7466386. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Richards H. H., Cantoni G. L. S-Adenosyl-L-homocysteine hydrolase: analogues of S-adenosyl-L-homocysteine as potential inhibitors. Mol Pharmacol. 1977 Sep;13(5):939–947. [PubMed] [Google Scholar]
- Christman J. K., Price P., Pedrinan L., Acs G. Correlation between hypomethylation of DNA and expression of globin genes in Friend erythroleukemia cells. Eur J Biochem. 1977 Nov 15;81(1):53–61. doi: 10.1111/j.1432-1033.1977.tb11926.x. [DOI] [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Hoffman J. A rapid liquid chromatographic determination of S-adenosylhomocysteine in subgram amounts of tissue. Anal Biochem. 1975 Oct;68(2):522–530. doi: 10.1016/0003-2697(75)90647-8. [DOI] [PubMed] [Google Scholar]
- Hoffman T., Hirata F., Bougnoux P., Fraser B. A., Goldfarb R. H., Herberman R. B., Axelrod J. Phospholipid methylation and phospholipase A2 activation in cytotoxicity by human natural killer cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3839–3843. doi: 10.1073/pnas.78.6.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Chiang P. K., Cantoni G. L. The action of the adenosylhomocysteine hydrolase inhibitor, 3-deazaadenosine, on phagocytic function of mouse macrophages and human monocytes. Biochem Biophys Res Commun. 1978 Sep 14;84(1):102–109. doi: 10.1016/0006-291x(78)90269-3. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Noguchi C. T., Turner P. H., Schechter A. N., Heller P., Nienhuis A. W. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983 Aug;62(2):370–380. [PubMed] [Google Scholar]
- Montgomery J. A., Clayton S. J., Thomas H. J., Shannon W. M., Arnett G., Bodner A. J., Kion I. K., Cantoni G. L., Chiang P. K. Carbocyclic analogue of 3-deazaadenosine: a novel antiviral agent using S-adenosylhomocysteine hydrolase as a pharmacological target. J Med Chem. 1982 Jun;25(6):626–629. doi: 10.1021/jm00348a004. [DOI] [PubMed] [Google Scholar]
- Morita Y., Siraganian R. P. Inhibition of IgE-mediated histamine release from rat basophilic leukemia cells and rat mast cells by inhibitors of transmethylation. J Immunol. 1981 Oct;127(4):1339–1344. [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. P., Wolberg G., Duncan G. S. Inhibition of lymphocyte-mediated cytolysis by 3-deazaadenosine: evidence for a methylation reaction essential to cytolysis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6220–6224. doi: 10.1073/pnas.75.12.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]



