Abstract
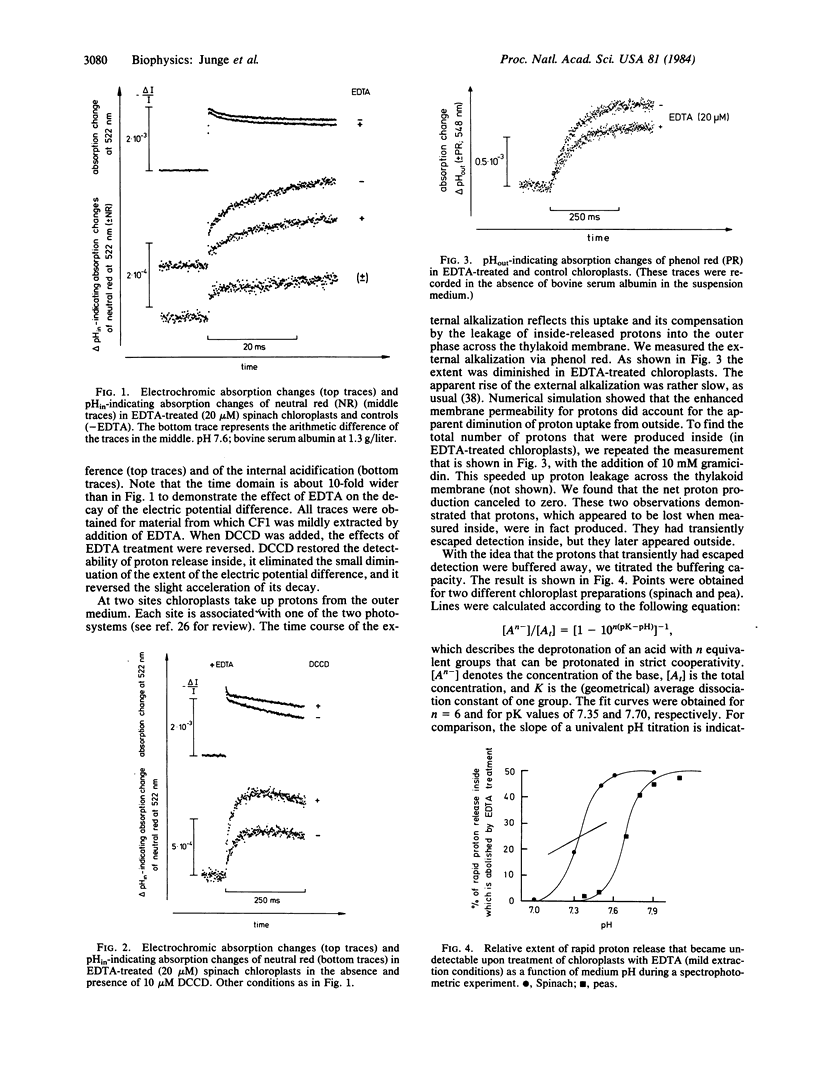
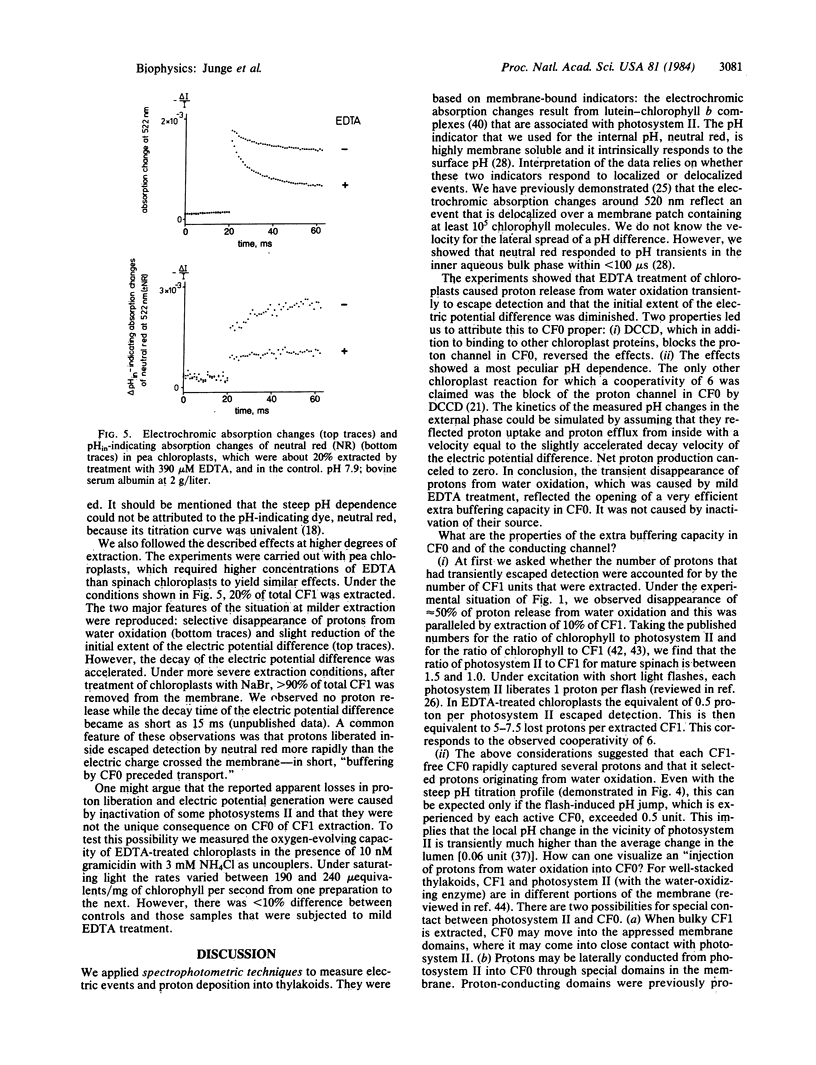
The ATP-synthase in chloroplasts is built from two blocks, CF0, which is integral to the thylakoid membrane and which serves as a proton channel, and CF1, attached to CF0, which is catalytically active. This study is aimed at understanding proton conduction through CF0. By a mild procedure we extracted <10% of total CF1, predominantly the four-subunit CF1 without the δ subunit. Extracted chloroplasts were excited with short flashes of light and the time course of the transmembrane potential and of the pH changes in both phases was measured spectrophotometrically. Mild extraction of CF1 caused two effects. (i) Up to 50% of the protons rapidly released from water oxidation transiently escaped detection in the thylakoid interior. (ii) The initial extent of the transmembrane potential was decreased by some 10% (20-μs resolution). Protons that were not detected inside appeared in the external phase after having passed the thylakoid membrane. pH titrations of the transient loss of protons produced an extremely sharp transition (near pH 7.5) as if six protons were buffered in a strictly cooperative manner. These effects were reversed upon addition of N,N′-dicyclohexylcarbodiimide, which, among other actions, blocks the proton channel through CF0. We interpret these observations as follows. (i) CF0 incorporates proton binding groups, which can act in a hexacooperative way. These groups are located near the middle of the membrane. (ii) After extraction of CF1, protons produced during water oxidation have very rapid access to these groups, but they pass the full span of the membrane more slowly: buffering precedes conduction through CF0.
Keywords: photosynthesis, water oxidation, photophosphorylation, proton pumping, proteolipid
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsson P. A., Andersson B., Larsson C., Akerlund H. E. Phase partition--a method for purification and analysis of cell organelles and membrane vesicles. Methods Biochem Anal. 1982;28:115–150. doi: 10.1002/9780470110485.ch2. [DOI] [PubMed] [Google Scholar]
- Andersson B., Anderson J. M. Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1980 Dec 3;593(2):427–440. doi: 10.1016/0005-2728(80)90078-x. [DOI] [PubMed] [Google Scholar]
- Andreo C. S., Patrie W. J., McCarty R. E. Effect of ATPase activation and the delta subunit of coupling factor 1 on reconstitution of photophosphorylation. J Biol Chem. 1982 Sep 10;257(17):9968–9975. [PubMed] [Google Scholar]
- Ausländer W., Junge W. The electric generator in the photosynthesis of green plants. II. Kinetic correlation between protolytic reactions and redox reactions. Biochim Biophys Acta. 1974 Aug 23;357(2):285–298. doi: 10.1016/0005-2728(74)90067-x. [DOI] [PubMed] [Google Scholar]
- Binder A., Jagendorf A., Ngo E. Isolation and composition of the subunits of spinach chloroplast coupling factor protein. J Biol Chem. 1978 May 10;253(9):3094–3100. [PubMed] [Google Scholar]
- Carlier M. F., Holowka D. A., Hammes G. G. Interaction of photoreactive and fluorescent nucleotides with chloroplast coupling factor 1. Biochemistry. 1979 Aug 7;18(16):3452–3457. doi: 10.1021/bi00583a003. [DOI] [PubMed] [Google Scholar]
- Deters D. W., Racker E., Nelson N., Nelson H. Partial resolution of the enzymes catalyzing photophosphorylation. XV. Approaches to the active site of coupling factor I. J Biol Chem. 1975 Feb 10;250(3):1041–1047. [PubMed] [Google Scholar]
- Junge W., Ausländer W., McGeer A. J., Runge T. The buffering capacity of the internal phase of thylakoids and the magnitude of the pH changes inside under flashing light. Biochim Biophys Acta. 1979 Apr 11;546(1):121–141. doi: 10.1016/0005-2728(79)90175-0. [DOI] [PubMed] [Google Scholar]
- Junge W., Witt H. T. On the ion transport system of photosynthesis--investigations on a molecular level. Z Naturforsch B. 1968 Feb;23(2):244–254. doi: 10.1515/znb-1968-0222. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- McCarty R. E., Fagan J. Light-stimulated incorporation of N-ethylmaleimide into coupling factor 1 in spinach chloroplasts. Biochemistry. 1973 Apr 10;12(8):1503–1507. doi: 10.1021/bi00732a006. [DOI] [PubMed] [Google Scholar]
- Merchant S., Shaner S. L., Selman B. R. Molecular weight and subunit stoichiometry of the chloroplast coupling factor 1 from Chlamydomonas reinhardi. J Biol Chem. 1983 Jan 25;258(2):1026–1031. [PubMed] [Google Scholar]
- Nelson N., Deters D. W., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of isolated subunits of coupling factor 1 from spinach chloroplasts. J Biol Chem. 1973 Mar 25;248(6):2049–2055. [PubMed] [Google Scholar]
- Nelson N., Eytan E., Notsani B. E., Sigrist H., Sigrist-Nelson K., Gitler C. Isolation of a chloroplast N,N'-dicyclohexylcarbodiimide-binding proteolipid, active in proton translocation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Schatz G. Biosynthesis and assembly of the proton-translocating adenosine triphosphatase complex from chloroplasts. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1361–1364. doi: 10.1073/pnas.77.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Oleszko S., Moudrianakis E. N. The visualization of the photosynthetic coupling factor in embedded spinach chloroplasts. J Cell Biol. 1974 Dec;63(3):936–948. doi: 10.1083/jcb.63.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Racker E. Purification and reconstitution of the N,N'-dicyclohexylcarbodiimide-sensitive ATPase complex from spinach chloroplasts. J Biol Chem. 1979 Apr 25;254(8):2793–2799. [PubMed] [Google Scholar]
- Prochaska L. J., Dilley R. A. Chloroplast membrane conformational changes measured by chemical modification. Arch Biochem Biophys. 1978 Apr 15;187(1):61–71. doi: 10.1016/0003-9861(78)90006-1. [DOI] [PubMed] [Google Scholar]
- Schindler H., Nelson N. Proteolipid of adenosinetriphosphatase from yeast mitochondria forms proton-selective channels in planar lipid bilayers. Biochemistry. 1982 Nov 9;21(23):5787–5794. doi: 10.1021/bi00266a010. [DOI] [PubMed] [Google Scholar]
- Schliephake W., Junge W., Witt H. T. Correlation between field formation, proton translocation, and the light reactions in photosynthesis. Z Naturforsch B. 1968 Dec;23(12):1571–1578. doi: 10.1515/znb-1968-1203. [DOI] [PubMed] [Google Scholar]
- Schmid R., Shavit N., Junge W. The coupling factor of photophosphorylation and the electric properties of the thylakoid membrane. Biochim Biophys Acta. 1976 Apr 9;430(1):145–153. doi: 10.1016/0005-2728(76)90230-9. [DOI] [PubMed] [Google Scholar]
- Shavit N. Energy transduction in chloroplasts: structure and function of the ATPase complex. Annu Rev Biochem. 1980;49:111–138. doi: 10.1146/annurev.bi.49.070180.000551. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Sigrist H., Azzi A. Characterization of the dicyclohexylcarbodiimide-binding protein isolated from chloroplast membranes. Eur J Biochem. 1978 Dec 1;92(1):9–14. doi: 10.1111/j.1432-1033.1978.tb12717.x. [DOI] [PubMed] [Google Scholar]
- Strotmann H., Bickel-Sandkötter S. Energy-dependent exchange of adenine nucleotides on chloroplast coupling factor (CF1). Biochim Biophys Acta. 1977 Apr 11;460(1):126–135. doi: 10.1016/0005-2728(77)90158-x. [DOI] [PubMed] [Google Scholar]
- Wagner R., Junge W. Gated proton conduction via the coupling factor of photophosphorylation modified by N,-N-orthophenyldimaleimide. Biochim Biophys Acta. 1977 Nov 17;462(2):259–272. doi: 10.1016/0005-2728(77)90124-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss M. A., McCarty R. E. Cross-linking within a subunit of coupling factor 1 increases the proton permeability of spinach chloroplast thylakoids. J Biol Chem. 1977 Nov 25;252(22):8007–8012. [PubMed] [Google Scholar]
- Witt H. T. Coupling of quanta, electrons, fields, ions and phosphrylation in the functional membrane of photosynthesis. Results by pulse spectroscopic methods. Q Rev Biophys. 1971 Nov;4(4):365–477. doi: 10.1017/s0033583500000834. [DOI] [PubMed] [Google Scholar]
- Younis H. M., Winget G. D., Racker E. Requirement of the delta subunit of chloroplast coupling factor 1 for photophosphorylation. J Biol Chem. 1977 Mar 10;252(5):1814–1818. [PubMed] [Google Scholar]



