Abstract
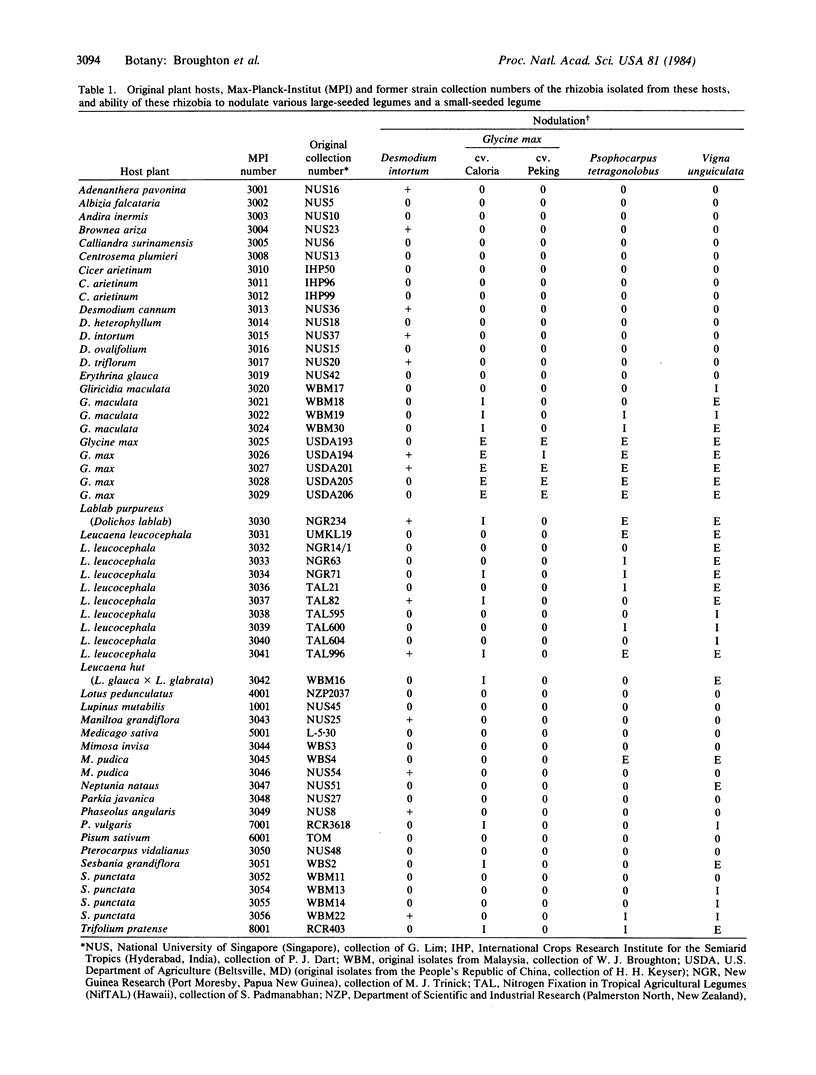
Forty-nine fast-growing Rhizobium strains from the nodules of 26 different tropical legume genera were screened to find isolates that would (i) nodulate, e.g., winged beans, so producing large nodules for RNA and protein isolation; (ii) also nodulate various small-seeded legumes, thus allowing screening of large numbers of mutants; and (iii) harbor plasmids containing nif structural genes as well as other functions involved in nodulation. On the basis of six different criteria, this rhizobial group appeared intermediate between classical fast- and slow-growing organisms, yet all contained plasmids. Plasmid numbers varied from one to five. Hybridizations between DNA prepared from nifDH and the putatative “nod” region of R. meliloti and these plasmids bound to nitrocellulose filters suggested that nif-nod genes are linked on a single sym plasmid. A broad-host-range strain containing a single sym plasmid was chosen for further study. Its plasmid, pMPIK3030a, was isolated on cesium chloride gradients and cloned in the cosmid pJB8, and the overlapping fragments were mapped by homology with the nif and nod regions of R. meliloti. As the wild-type plasmid pMPIK3030a was not self-transmissible, confirmation that the nod genes detected by homology were responsible for nodulation was obtained by introducing the mobilization functions of RP4 (together with Tn5) and selecting transconjugants resistant to kanamycin and neomycin. Transconjugants (obtained at a frequency of about 10-6 per recipient) in Agrobacterium tumefaciens cured of the Ti plasmid produced ineffective nodules on Vigna unguiculata, those in nonnodulating (Nod-) R. meliloti were partially effective, while those in Nod-R. leguminosarum were often fully effective.
Keywords: broad host range, recombinant DNA, plasmid mobilization, effective transconjugants
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broughton W. J., Dilworth M. J. Control of leghaemoglobin synthesis in snake beans. Biochem J. 1971 Dec;125(4):1075–1080. doi: 10.1042/bj1251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Elmerich C., Dreyfus B. L., Reysset G., Aubert J. P. Genetic analysis of nitrogen fixation in a tropical fast-growing Rhizobium. EMBO J. 1982;1(4):499–503. doi: 10.1002/j.1460-2075.1982.tb01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Edmiston S. H., Harper J., Mount D. W. Isolation and characterization of an operator-constitutive mutation in the recA gene of E. coli K-12. Mol Gen Genet. 1982;187(1):4–11. doi: 10.1007/BF00384376. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. D., ALLEN O. N. Cultural reactions of rhizobia with special reference to strains isolated from Sesbania species. Antonie Van Leeuwenhoek. 1952;18(1):1–12. doi: 10.1007/BF02538585. [DOI] [PubMed] [Google Scholar]
- JOHNSON M. D., ALLEN O. N. Nodulation studies with special reference to strains isolated from Sesbania species. Antonie Van Leeuwenhoek. 1952;18(1):12–22. [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- Masterson R. V., Russell P. R., Atherly A. G. Nitrogen fixation (nif) genes and large plasmids of Rhizobium japonicum. J Bacteriol. 1982 Nov;152(2):928–931. doi: 10.1128/jb.152.2.928-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison N. A., Hau C. Y., Trinick M. J., Shine J., Rolfe B. G. Heat curing of a sym plasmid in a fast-growing Rhizobium sp. that is able to nodulate legumes and the nonlegume Parasponia sp. J Bacteriol. 1983 Jan;153(1):527–531. doi: 10.1128/jb.153.1.527-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]






