Abstract
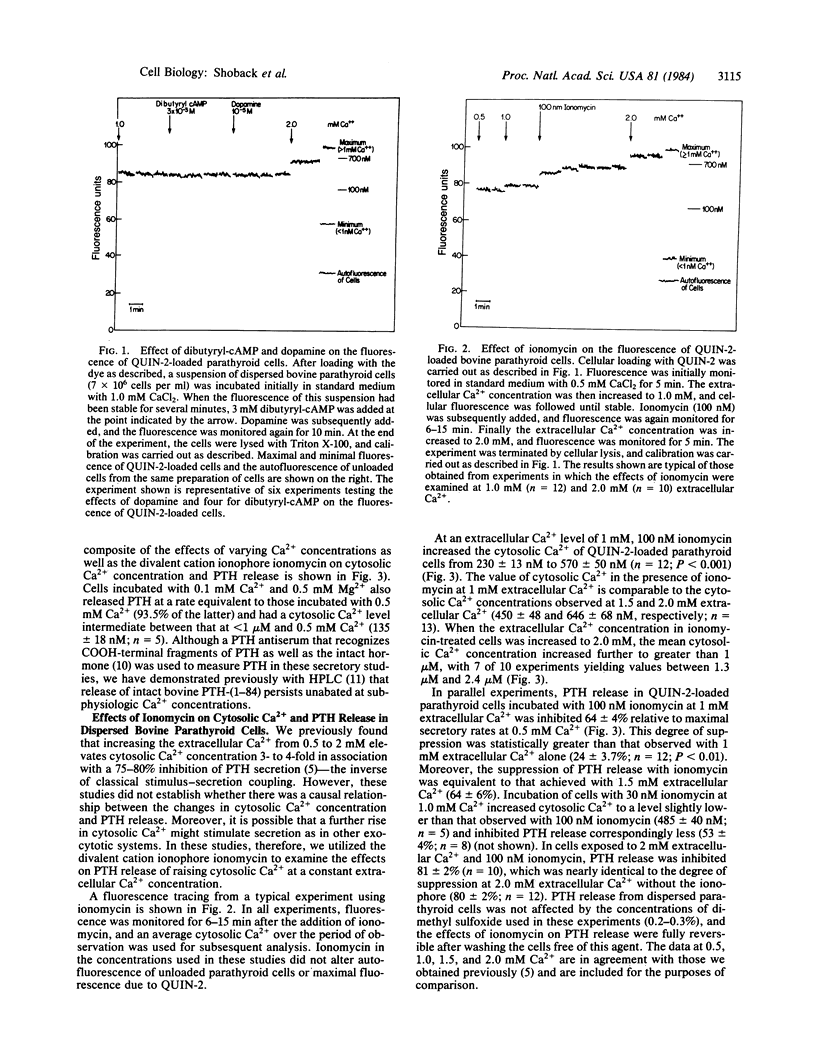
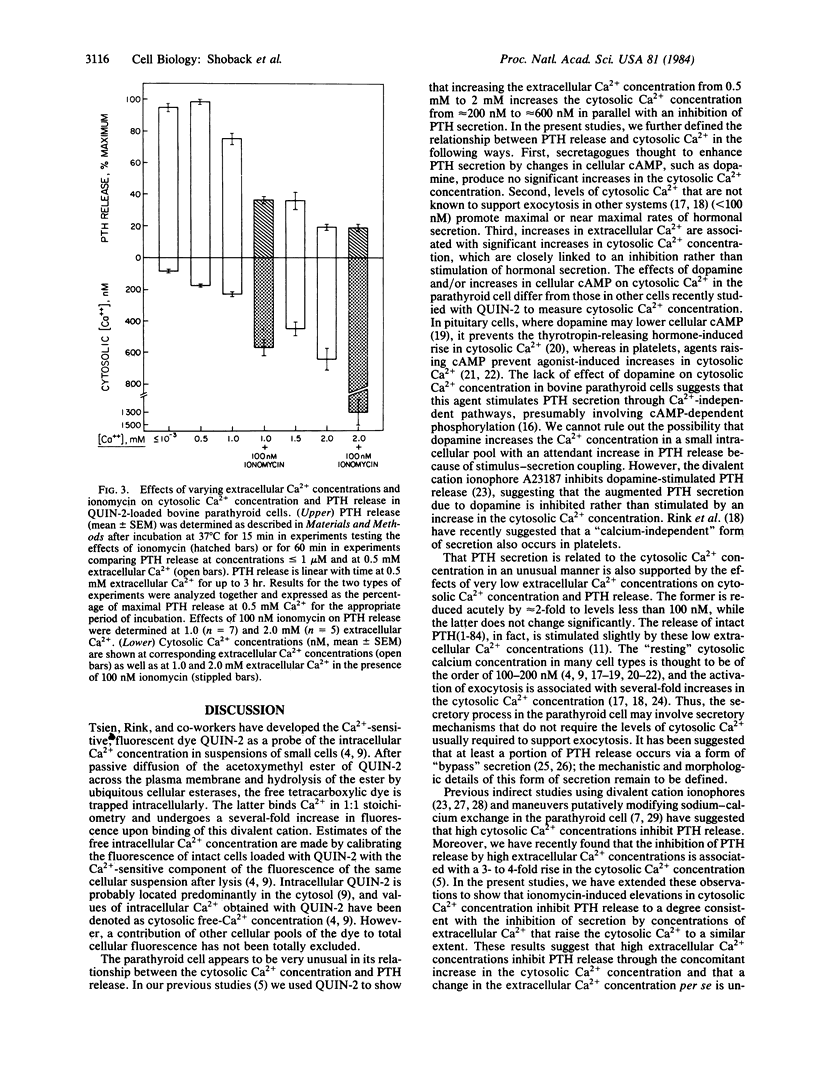
The parathyroid cell is unusual among exocytotic systems in that low extracellular Ca2+ concentrations stimulate, while high Ca2+ concentrations inhibit, parathyroid hormone (PTH) release, suggesting that this cell might have unique secretory mechanisms. In the present studies, we used the Ca2+-sensitive fluorescent dye QUIN -2 to examine the relationship between cytosolic Ca2+ concentration and PTH release in dispersed bovine parathyroid cells. The secretagogue dopamine, which enhances PTH release 2- to 3-fold in association with 20- to 30-fold increases in cellular cAMP, had no effect on the cytosolic Ca2+ level (261 +/- 28 vs. 236 +/- 22 nM for control cells at 1 mM extracellular Ca2+; P greater than 0.05). Dibutyryl-cAMP, which produces a comparable stimulation of PTH release, likewise did not modify the level of cytosolic Ca2+. Removal of extracellular Ca2+ produced a further decrease of the cytosolic Ca2+ to 82 +/- 10 nM. However, PTH secretion persisted at a near maximal rate despite this decrease of extracellular and cytosolic Ca2+ and was 95 +/- 2.5% of the rate of hormonal release at 0.5 mM extracellular Ca2+. In contrast, addition of the divalent cation ionophore ionomycin to parathyroid cells at 1.0 mM extracellular Ca2+ inhibited PTH secretion in association with an increase in cytosolic Ca2+ from 230 +/- 13 nM to 570 +/- 50 nM. Moreover, the magnitude of the ionomycin-induced reduction in PTH secretion (64 +/- 4% relative to the secretory rate at 0.5 mM Ca2+) was equivalent to the inhibition of PTH release caused by 1.5 mM extracellular Ca2+ (64 +/- 6%), which increased the cytosolic Ca2+ to similar levels (450 +/- 48 nM). Thus, the parathyroid cell differs from secretory cells thought to operate by stimulus-secretion coupling in the following ways: changes in PTH release can occur without detectable alterations in the cytosolic Ca2+ concentration, maximal rates of PTH secretion occur at cytosolic Ca2+ concentrations that fail to support exocytosis in other cell types, and increases in the cytosolic Ca2+ concentration due to ionomycin inhibit rather than stimulate PTH release. Therefore, the control of PTH secretion by Ca2+ and other secretagogues may involve previously undefined mechanisms whereby hormonal release is relatively independent of the cytosolic Ca2+ at low levels of this parameter and is inversely related to cytosolic Ca2+ at higher levels of intracellular Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Knight D. E. Gaining access to the site of exocytosis in bovine adrenal medullary cells. J Physiol (Paris) 1980 Sep;76(5):497–504. [PubMed] [Google Scholar]
- Brown E. M., Adragna N., Gardner D. G. Effect of potassium on PTH secretion from dispersed bovine parathyroid cells. J Clin Endocrinol Metab. 1981 Dec;53(6):1304–1306. doi: 10.1210/jcem-53-6-1304. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Carroll R. J., Aurbach G. D. Dopaminergic stimulation of cyclic AMP accumulation and parathyroid hormone release from dispersed bovine parathyroid cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4210–4213. doi: 10.1073/pnas.74.10.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. M., Gardner D. G., Aurbach G. D. Effects of the calcium ionophore A23187 on dispersed bovine parathyroid cells. Endocrinology. 1980 Jan;106(1):133–138. doi: 10.1210/endo-106-1-133. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Hurwitz S., Aurbach G. D. Preparation of viable isolated bovine parathyroid cells. Endocrinology. 1976 Dec;99(6):1582–1588. doi: 10.1210/endo-99-6-1582. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Thatcher J. G. Adenosine 3',5'-monophosphate (cAMP)-dependent protein kinase and the regulation of parathyroid hormone release by divalent cations and agents elevating cellular cAMP in dispersed bovine parathyroid cells. Endocrinology. 1982 Apr;110(4):1374–1380. doi: 10.1210/endo-110-4-1374. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Watson E. J., Leombruno R., Underwood R. H. Extracellular calcium is not necessary for acute, low calcium- or dopamine-stimulated PTH secretion in dispersed bovine parathyroid cells. Metabolism. 1983 Nov;32(11):1038–1044. doi: 10.1016/0026-0495(83)90074-4. [DOI] [PubMed] [Google Scholar]
- Care A. D., Sherwood L. M., Potts J. T., Jr, Aurbach G. D. Perfusion of the isolated parathyroid gland of the goat and sheep. Nature. 1966 Jan 1;209(5018):55–57. doi: 10.1038/209055a0. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Macconi D., Spada A. Dopamine inhibits adenylate cyclase in human prolactin-secreting pituitary adenomas. Nature. 1979 Mar 15;278(5701):252–254. doi: 10.1038/278252a0. [DOI] [PubMed] [Google Scholar]
- Dietel M., Dorn-Quint G. By-pass secretion of human parathyroid adenomas. A particular intracellular form of rapid adaptation to external stimuli. Lab Invest. 1980 Aug;43(2):116–125. [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: variations on the theme of calcium-activated exocytosis involving cellular and extracellular sources of calcium. Ciba Found Symp. 1978;(54):61–90. doi: 10.1002/9780470720356.ch4. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Egan J. J., Sha'afi R. I., White J. The cytoplasmic concentration of free calcium in platelets is controlled by stimulators of cyclic AMP production (PGD2, PGE1, forskolin). Biochem Biophys Res Commun. 1983 Jun 15;113(2):598–604. doi: 10.1016/0006-291x(83)91768-0. [DOI] [PubMed] [Google Scholar]
- Gardner D. G., Brown E. M., Aurbach G. D. Inhibition of parathyroid hormone release from dispersed bovine cells by ouabain. Metabolism. 1983 Apr;32(4):355–358. doi: 10.1016/0026-0495(83)90043-4. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Stevens T. D., Ravazzola M., Orci L., Potts J. T., Jr Effects of calcium ionophores on the synthesis and release of parathyroid hormone. Endocrinology. 1977 Nov;101(5):1524–1537. doi: 10.1210/endo-101-5-1524. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Kesteven N. T. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):177–199. doi: 10.1098/rspb.1983.0033. [DOI] [PubMed] [Google Scholar]
- Lasker R. D., Spiegel A. M. Endogenous substrates for cAMP-dependent phosphorylation in dispersed bovine parathyroid cells. Endocrinology. 1982 Oct;111(4):1412–1414. doi: 10.1210/endo-111-4-1412. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Hamilton J. W., Cohn D. V. The by-pass of tissue hormone stores during the secretion of newly synthesized parathyroid hormone. Endocrinology. 1975 Jul;97(1):178–188. doi: 10.1210/endo-97-1-178. [DOI] [PubMed] [Google Scholar]
- McGregor D. H., Morrissey J. J., Cohn D. V. The effects of tris(hydroxymethyl)aminomethane and calcium ionophores on the biosynthesis of proparathormone and the formation and degradation of parathormone in bovine parathyroid tissue. Am J Pathol. 1981 Mar;102(3):336–345. [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. J., Hamilton J. W., MacGregor R. R., Cohn D. V. The secretion of parathormone fragments 34-84 and 37-84 by dispersed porcine parathyroid cells. Endocrinology. 1980 Jul;107(1):164–171. doi: 10.1210/endo-107-1-164. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Rothstein M., Morrissey J., Slatopolsky E., Klahr S. The role of Na+-Ca++ exchange in parathyroid hormone secretion. Endocrinology. 1982 Jul;111(1):225–230. doi: 10.1210/endo-111-1-225. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Schofield J. G. Use of a trapped fluorescent indicator to demonstrate effects of thyroliberin and dopamine on cytoplasmic calcium concentrations in bovine anterior pituitary cells. FEBS Lett. 1983 Aug 8;159(1-2):79–82. doi: 10.1016/0014-5793(83)80420-7. [DOI] [PubMed] [Google Scholar]
- Shoback D., Thatcher J., Leombruno R., Brown E. Effects of extracellular Ca++ and Mg++ on cytosolic Ca++ and PTH release in dispersed bovine parathyroid cells. Endocrinology. 1983 Jul;113(1):424–426. doi: 10.1210/endo-113-1-424. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Wallace J., Scarpa A. Regulation of parathyroid hormone secretion in vitro by divalent cations and cellular metabolism. J Biol Chem. 1982 Sep 25;257(18):10613–10616. [PubMed] [Google Scholar]
- Williams G. A., Hargis G. K., Bowser E. N., Henderson W. J., Martinez N. J. Evidence for a role of adenosine 3',5'-monophosphate in parathyroid hormone release. Endocrinology. 1973 Mar;92(3):687–691. doi: 10.1210/endo-92-3-687. [DOI] [PubMed] [Google Scholar]



