Abstract
Anti-angiogenic therapies, including bevacizumab, are being used with increasing frequency in the management of malignant glioma. Common clinically significant toxicities include hypertension and proteinuria, poor wound healing, and the potential for thromboembolic events. Literature related to the use of bevacizumab in malignant glioma, reported toxicities in this patient population, and management of these toxicities was reviewed. Recommendations for assessment and management are provided. Anti-angiogenic therapies will continue to have a role in the treatment of malignant glioma. Further studies of the prevention, assessment, and management of these toxicities are warranted.
Keywords: brain tumors, chemotherapy, toxicity
Malignant gliomas remain difficult to treat, and standard approaches are associated with poor survival. Despite level 1 evidence of a survival benefit for those patients treated with concurrent temozolomide and radiation, followed by adjuvant temozolomide in patients with glioblastoma (GBM), the overall median survival, even with this therapy, was only 14.6 months.1 Even for patients who receive a diagnosis of anaplastic gliomas (World Health Organization [WHO] grade 3), median survival is only 2–4 years.2–4 For all high-grade gliomas, achieving response after the tumor has recurred is difficult and survival is poor. In recurrent GBM, using temozolomide, response rates of 5% and 6-month progression-free survival of 21% have been reported.5,6 Therefore, effective salvage therapies for patients with GBM are needed.
Angiogenesis and Tumor Growth
Previous preclinical and clinical investigations have established that most solid tumor growth beyond several millimeters is dependent on angiogenesis.7,8 Angiogenesis is a physiologic process involving a balance of angiogenic factors and inhibitors that control microvessel sprout growth and proliferation of endothelial cells.9 The importance of this increased vasculature in glioma was first observed by Virchow during the 19th century10,11 and was recognized to be integral to tumor growth. The profound importance of angiogenesis in brain tumor biology is highlighted by the recognition that endothelial proliferation is a hallmark of GBM and is considered to be a major criterion in conferring the histopathological diagnosis. GBM cells are known to produce angiogenic factors, such as basic fibroblast growth factor, hepatocyte growth factor/scatter factor, and vascular endothelial growth factor-A (VEGF-A).11–13 In addition, endothelial cells in tumor express VEGFR2 (KDR), resulting in a paracrine loop. These signaling pathways work with other important pathways and glioma stem-like cells to result in new vessel formation supporting continued tumor growth.
Anti-Angiogenic Therapy
Because of the importance of vascular proliferation in the biology of GBM, targeting angiogenesis may be an important treatment for malignant gliomas. Several agents are under investigation, targeting different components of the angiogenesis pathway. There are several potential therapeutic advantages of targeting angiogenesis in the treatment of malignant gliomas. These include tumor selectivity because of selective vulnerability of the newly formed vasculature; less concern about drug delivery as the primary targets of these therapies because the endothelial cells are in the microvascular niche, which is outside of the blood-brain barrier; and minimal myelotoxicity permitting combination with conventional cytotoxic agents (although other systemic toxicities have been reported).
The first anti-angiogenic inhibitor approved for clinical use in brain tumors is bevacizumab, a recombinant humanized IgG1 monoclonal antibody.14,15 Bevacizumab was first approved in 2004 as first-line treatment for metastatic colorectal cancer in combination with conventional cytotoxic chemotherapy. Later, bevacizumab was approved for the treatment of lung and breast cancer in combination with cytotoxic chemotherapy and with interferon in renal cancer. In malignant gliomas, there are several retrospective and prospective trials in patients with recurrent GBM.16–25 Two pivotal prospective trials, AVG3708g, an open-label multicenter trial and, NCI 06-C-0064E, led to accelerated approval by the United States Food and Drug Administration for use in brain tumors in May 2009 as a monotherapy in patients with GBM who progressed after first-line therapy. Recent and ongoing phase II and phase III trials are evaluating use of bevacizumab both in the up-front setting and in combination with a variety of agents for recurrent disease.
Although the clinical trials demonstrate the apparent efficacy of bevacizumab, there are several issues related to the administration of anti-angiogenic agents that may complicate their use in the population with malignant glioma. Concerns have been raised regarding the clinical significance of reduction of contrast enhancement, the standard metric of objective response that can occur within hours or days after administration of anti-angiogenic agents (i.e., bevacizumab and cediranib). It remains uncertain whether the reduction in contrast enhancement is the result of true tumor response, treatment-induced reduction of blood-brain barrier permeability, or a combination of both.26 Preclinical models suggest that alterations in blood-brain barrier permeability may be the dominant effect.27 Furthermore, there is concern that the use of anti-angiogenic agents may change tumor biology, resulting in alterations in pattern of tumor spread to a more infiltrative pattern that is highly refractory to salvage treatments.
In addition to the aforementioned issues related to the use of anti-angiogenic agents, treatment-associated toxicities further complicate care. Common toxicities include hypertension and proteinuria, poor wound healing, and the increased risk for venous and arterial thromboembolic events. These toxicities have specific implications for patients with brain tumor because of the inherent increased risk of thrombosis and issues with wound healing related to chronic corticosteroid use. These toxicities further complicate care by influencing further neurosurgical intervention and tolerance of other therapies. Understanding the incidence and diagnostic and management strategies for toxicities from anti-angiogenic therapy for the population with malignant glioma is an important component of care.
Hypertension
The mechanism of arterial hypertension associated with anti-VEGF therapy is complex and almost certainly multifactorial. Nitric oxide, which helps maintain the balance between vasoconstriction and vasodilation, is a major contributor. VEGF normally increases endothelial transcription of nitric oxide synthase, and anti-VEGF agents decrease nitric oxide production, resulting in vasoconstriction.28,29 At the renal level, this vasoconstriction produces sodium retention, adding further to hypertension.28 A reduction in density of microvascular beds, a phenomenon termed “rarefaction,” increasing systemic vascular resistance and blood pressure may be a second mechanism.28 Recent human studies support bevacizumab- and sunitinib-mediated rarefaction as a component of hypertension.30,31 Finally, endothelial oxidative stress has been implicated as a factor in the development of hypertension, and upregulation of VEGF and VEGFR-2 plays a role in protecting the endothelium from reactive oxygen species. Therefore, treatment-induced diminution of this protective mechanism may predispose to hypertension.28
Bevacizumab treatment is associated with a high incidence of hypertension. However, it is important to recognize that most published studies reporting on hypertension with anti-VEGF agents have used Common Terminology Criteria for Adverse Events (CTCAE), version 2.0 or 3.0 and not version 4.0, which was recently modified to bring their criteria into line with the Seventh Report of the Joint National Commission on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7).32 A meta-analysis of clinical trials that randomized patients with multiple tumor types to chemotherapy with or without bevacizumab determined that low-dose (<10 mg/kg/dose) bevacizumab increased the incidence of any grade hypertension by 3-fold and that high-dose (≥10 mg/kg/dose) bevacizumab increased such incidence by 7.5-fold.33 A subsequent meta-analysis found that 24% of patients receiving bevacizumab developed any grade of hypertension and 8% developed grade 3+ hypertension (relative risk [RR], 5.3).34 The incidence of bevacizumab-associated hypertension in glioblastoma appears to be similar; the BRAIN study reported an all-grade rate of 31% and grade 3+ hypertension in 5% of patients.20 VEGFR tyrosine kinase inhibitors (TKIs) induce hypertension at similar rates, with figures of 22% and 23% for sunitinib and sorafenib.35,36 Risk factors for development of hypertension with bevacizumab remain to be elucidated.29,37
Although long-term hypertension is an important risk factor for the development of coronary artery and chronic renal disease, ischemic stroke, and intracranial hemorrhage, the typically limited life expectancy of patients with malignant glioma who are receiving anti-VEGF therapy may render these complications as secondary concerns in treatment decision-making. Nonetheless, there are compelling reasons to treat hypertension. In some patients, blood pressure elevation is dramatic and, if untreated, may preclude continued anti-VEGF agent administration. Moreover, hypertension-related adverse events sometimes have a rapid onset with anti-VEGF therapy. Finally, a modest but increasing percentage of patients with glioblastoma become long-term survivors and, therefore, more vulnerable to the chronic effects of hypertension. Consequently, determination of the cardiovascular risk of hypertension in a given patient is advised, including assessment of risk factors, such as diabetes mellitus, known cardiovascular disease, chronic kidney disease, tobacco use, hyperlipidemia, obesity, family history, and advanced age. In patients with low cardiovascular risk, the treatment goal is 140/90 mm Hg; in high-risk patients, it is 130/80 mm Hg.38
The management of hypertension associated with bevacizumab and other anti-VEGFR agents follows the principles of hypertension management in general. Other risk factors for developing hypertension should be addressed, including reduction of salt intake and avoidance of excessive alcohol, nonsteroidal anti-inflammatory drugs, sympathomimetics, oral contraceptives, and corticosteroids when possible.38,39 A single antihypertensive agent is typically sufficient to control blood pressure. There are no controlled studies to support use of a specific agent or class of agents. Nonetheless, some considerations are pertinent. For example, among calcium channel blockers, diltiazam and verapamil inhibit CYP 3A4 and nifedipine induces VEGF. Because amlodipine and felodipine do not share the potential for major drug interactions, these newer agents are preferred.39 Angiotensin-converting enzyme (ACE) inhibitors work more quickly than calcium channel blockers and should be considered when rapid treatment is desirable; in addition, they are the preferred treatment in the context of proteinuria. Table 1 summarizes some other considerations in choice of class of anti-hypertensive agent. Monitoring blood pressure weekly during the first bevacizumab cycle and then with each subsequent infusion is advisable. Patients with essential hypertension are encouraged to monitor blood pressure at home.29
Table 1.
Consideration in choice of antihypertensive agents
| Class of Drug | Cancer-specific cautions or reasons to avoid | Basis for preferred selection | General cautions and contraindications |
|---|---|---|---|
| Angiotensin-converting enzyme inhibitors | Coadministration/titration with renal clearance-dependent agents (e.g. cisplatin and pemetrexed); hyperkalemia | Left ventricular systolic dysfunction; diabetic nephropathy | Renovascular disease; peripheral vascular disease; renal impairment |
| Angiotensin II receptor blockers | Coadministration/titration with renal clearance-dependent agents (e.g., cisplatin and pemetrexed); hyperkalemia | Intolerance of other agents, especially ACE inhibitors; left ventricular systolic dysfunction; diabetic nephropathy | Renovascular disease; peripheral vascular disease; renal impairment |
| Beta blockers | Asthenia; malaise; fatigue; QT interval prolonging drugs | Angina; history of myocardial infarction; anxiety | Bradycardia/heart clock; diabetes (risk for hypoglycemia); asthma/chronic obstructive pulmonary disease (wheezing)’ decompensated heart failure |
| Calcium channel blockers (e.g., dihydropyridines) | Lower extremity swelling | Elderly patients; isolated systolic hypertension | Preexisting edema; slow onset of action |
| Thiazide diuretics | Gout; hypercalcemia; hypokalemia; young patients (age ≤45 yr); QT interval prolonging drugs | Elderly patients; isolated systolic hypertension secondary stroke prevention; typically least expensive | Gout; documented sulfa allergy |
Reprinted from open access: Maitland and colleagues.47
Proteinuria
The kidneys normally prevent significant loss of plasma proteins in the urine. Glomeruli act as molecular sieves, retaining high–molecular weight proteins, and low–molecular weight proteins filtered into the tubular lumen are reabsorbed in the proximal tubule. Several studies have noted that hypertension is a strong risk factor for bevacizumab-associated proteinuria,33,40 leading to the hypothesis that bevacizumab-induced hypertension produces proteinuria by increasing intraglomerular pressure.33
Although hypertension likely contributes to proteinuria, the role of VEGF in maintaining glomerular integrity represents another important mechanism. Podocytes, a key constituent of the glomerular filtration mechanism, constitutively express VEGF; moreover, VEGF receptors are present on glomerular capillary endothelial cells.41 In mice, targeted heterozygous deletion or pharmacologic inhibition of VEGF in podocytes produces renal injury, including loss of endothelial fenestrations and proteinuria.42 The thrombotic microangiopathy that these animals develop precedes the onset of hypertension. Similarly, renal biopsy specimens from several patients who developed proteinuria while receiving bevacizumab revealed a consistent pattern of thrombotic microangiopathy.42 Renal thrombotic microangiopathy with proteinuria has also been reported with the VEGFR TKIs sunitinib43 and sorafenib.44
Normal urine protein excretion is 40–80 mg daily, and levels >150 mg daily are considered to be abnormal. There are several ways to measure urinary protein excretion. Historically, a 24-hour timed urine collection for assessment of total albumin or protein was traditional. However, in addition to their inconvenience, such measurements have a surprisingly high coefficient of variation (up to 20%).45 Single-voided specimens are far more convenient but vary substantially in terms of urine protein concentration. Because urinary creatinine is a marker of urine concentration, the ratio of urine protein to urine creatinine (UPC) provides an estimate of urine protein that controls for concentration. Fortuitously, because daily urine creatinine production for an average-sized adult is ∼1000 mg, a spot UPC ratio assessment approximates 24 h urine protein excretion in grams. A normal UPC ratio is <0.2. Finally, urine dipsticks detect albuminuria; however, their dependence on urine concentration, relative insensitivity for nonalbumin proteins, and lack of specificity make them a better screening tool than means of quantifying and following proteinuria. However, dipstick-determined proteinuria is not a substitute for the more quantitative UPC ratio.
The National Cancer Institute CTCAE, version 4.0, grades proteinuria in adults from 1 to 3. Grade 1 proteinuria corresponds to a 1+ urine dipstick with 24-hour urine protein level <1.0 g. A 2+ urine dipstick or 24-hour protein level of 1.0–3.4 g qualifies as grade 2, and 24-hour urine protein level ≥3.5 g constitutes grade 3 proteinuria. A recent meta-analysis of several randomized trials with and without bevacizumab has quantified the risk of proteinuria.46 Thirteen percent of patients receiving bevacizumab had at least grade 1 proteinuria, and 2.2% had grade 3+ proteinuria (a 5-fold increase, compared with patients receiving chemotherapy without bevacizumab). High-grade proteinuria is a dose-limiting toxicity for another VEGF-sequestering agent, aflibercept (VEGF Trap).47 Corresponding data for VEGFR-targeting TKIs are more elusive, although phase II axitinib trials reported all-grade proteinuria rates of 18%–36% and grade 3+ rates of 0%–5%.41 Similarly, 30% of women in a phase II study of cediranib for ovarian cancer developed grade 1 or 2 proteinuria at a median of 2 weeks after starting therapy;48 median time to development of proteinuria with bevacizumab in glioblastoma has not been reported.
The incidence of bevacizumab-related proteinuria appears to be lower in patients with glioblastoma than other cancers. The BRAIN study found grade 1 proteinuria in only 4% and grade 3 proteinuria in just 1 of 167 patients.20 None of the 48 patients treated in the National Institutes of Health bevacizumab study were reported to develop proteinuria.21 Why proteinuria is less common in brain tumor patients has not been examined, although a shorter median duration of therapy may play a role.
Patients receiving bevacizumab require periodic monitoring for development of proteinuria. The package insert recommends dipstick of serial urinalyses, and because of the relative infrequence of clinically significant proteinuria in glioblastoma (particularly in the context of recurrent tumor), monitoring every other infusion seems to be reasonable.41 According to the manufacturer, a urine dipstick ≥2+ warrants further assessment with a 24-hour urine collection for protein (http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf); permanent discontinuation of bevacizumab is recommended for nephrotic syndrome and temporary suspension for 24 h urine protein totaling >2 g, with resumption when <2 g. Our practice has been to follow the UPC ratio after the urine dipstick shows 2+ proteinuria. There are no suggested bevacizumab dose modifications for patients with renal dysfunction.
Beyond holding or discontinuing drug, management of bevacizumab-associated proteinuria remains uncertain. For proteinuria in general, ACE inhibitors and angiotensin II receptor blocks (ARBs) reduce the severity of the proteinuria and the risk of end-stage renal disease beyond their impact when controlling hypertension. Despite the relative frequency of anti-VEGF agent-induced proteinuria, no interventional studies have been conducted, thus precluding evidence-based treatment recommendations.41 Nonetheless, ACE inhibitors or ARBs have been shown to reduce proteinuria in patients treated with mTOR inhibitors, and their renoprotective effects might be useful in patients with mild proteinuria. In patients with both hypertension and proteinuria, agents from these classes represent a rational first choice.
Reversible Posterior Leukoencephalopathy Syndrome
Reversible posterior leukoencephalopathy syndrome (RPLS), also known as posterior reversible encephalopathy syndrome, represents a neurological condition associated both with VEGF-sequestering agents and with TKIs targeting VEGFR. The clinical syndrome typically consists of the relatively acute onset of headaches, seizures, confusion, and often, cortical blindness. Most patients are markedly hypertensive. MRI typically reveals T2/FLAIR hyperintensities predominating in the white matter (Fig. 1). The lesions are typically more prominent in the posterior cerebral hemispheres but may involve anterior regions and posterior fossa structures. Contrast enhancement is variable but usually absent. The 2 principal theories relating to pathogenesis implicate failure of cerebral vasomotor autoregulation because of hypertension or primary endothelial damage (akin to pre-eclampsia). Anti-VEGF agents are capable of producing both of these mechanisms, and this may help explain why both bevacizumab and VEGFR TKIs have been implicated in RPLS.49,50 RPLS usually resolves quickly with treatment of hypertension and removal of the offending agent; subsequent reintroduction of an anti-VEGF agent is generally discouraged, although a recent case report noted successful resumption of bevacizumab post-RPLS (PMID 21900098).51
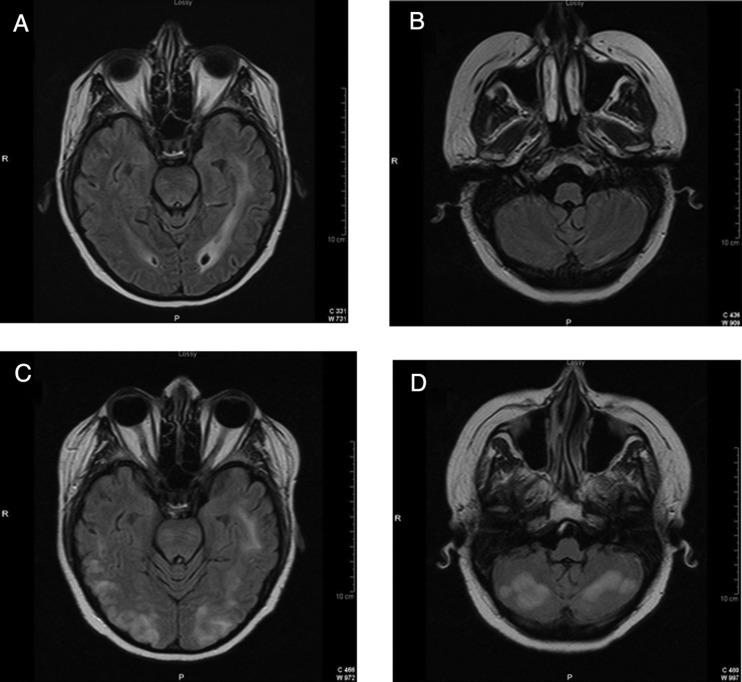
Fig. 1.
Reversible posterior leukoencephalopathy syndrome (RPLS): This 47-year-old woman had an 18-month history of a corpus callosum glioblastoma that progressed despite radiation with temozolomide, temsirolimus with sorafenib, and then BCNU (Carmustine). She presented 3 weeks after starting bevacizumab with headache, seizures, unresponsiveness, and blood pressure of 160/100 mm Hg. FLAIR-weighted MRI sequences 2 weeks before (A and B) and 3 weeks after her first dose of bevacizumab (C and D).
Hemorrhage
Anti-angiogenic agents are associated with an increased risk of both systemic and intracerebral hemorrhage (Table 2). Although an early meta-analysis of randomized controlled studies of bevacizumab failed to demonstrate a significant increase in the risk of hemorrhage,52 a more recent meta-analysis involving 12 617 patients from 20 randomized trials suggested that bevacizumab was associated with an increased risk of bleeding.53 Overall, high-grade hemorrhages (grades 3–5) occurred in 3.5% of patients. Bevacizumab was associated with an increased the risk of bleeding with an RR of 2.48 (95% confidence interval [CI], 1.93–3.18), compared with the controls. This risk was greater for patients treated with higher doses of bevacizumab (5 mg/kg/week; RR, 3.02; 95% CI, 2.42–3.78), compared with those receiving lower doses of bevacizumab (2.5 mg/kg/week; RR, 2.01; 95% CI, 1.43–2.83). Most hemorrhages occurred within the first 5 months of treatment. The most common type of hemorrhage is epistaxis, but more serious forms of bleeding, such as hemoptysis, gastrointestinal bleeding, intracerebral hemorrhage, and intratumoral hemorrhage, may also occur.
Table 2.
Incidence of thrombotic events and CNS hemorrhage from anti-VEGF therapies in patients with malignant gliomas
| Study | Agent(s) | Number of patients | Number with DVT/PE | Number with CNS or Systemic Bleed | Number with stroke |
|---|---|---|---|---|---|
| Vredenburgh, JCO 200724 | Bev/CPT | 35 | 4 (11%) | 1 (0.03%) | 0 |
| Vredenburgh, Clin Cancer Res59 | Bev/CPT | 32 | 3 (9%) | 0 | 1 (3%) |
| Friedman20 | Bev & Bev/CPT | 167 | 11 (7%) | 5 (3%) | 9 (5%) |
| Sathornsumetee73 | Bev/Erlotinib | 57 | 3 (5%) | 1 (2%) | 1 (2%) |
| Kreisl85 | Bev & Bev/CPT | 48 | 6 (8%) | 0 | 0 |
| Gilbert55 | Bev/CPT, Bev/TMZ | 117 | 14 (12%) | 9 (8%) 8 grade 1 or 2 | 1 (1%) |
| Lai et al.17 | Bev | 70 | 13 (19%) | 2 (3%)CNS, 2(3%) GI | 6 (9%) |
| Batchelor58 | Cediranib | 31 | 1 (1%) | 0 | 0 |
| Wen57 | XL184 | 153 | 15 (10%) | 7 (5%) | 0 |
VEGFR inhibitors are also associated with an increased risk of hemorrhage. One meta-analysis examining the use of sorafenib and sunitinib in 6799 patients with a variety of cancers found an incidence of high-grade bleeding of 2.4% (95% CI, 1.6–3.9) and an RR of 2.0 (95% CI, 1.14–3.49).54
The underlying mechanisms for the increased bleeding risk are complex and include damage of vascular integrity by the inhibition of endothelial survival and proliferation, particularly in tissues with a high VEGF dependence such as injured mucosal membrane of the airway; dysregulation of the coagulation cascade; damage to the tumor infiltrated vascular wall as a consequence of an antitumor effect; decreased matrix deposition in the supporting layers of vessels; and occasionally, treatment-induced thrombocytopenia.53
Low-grade systemic hemorrhages, such as epistaxis, are relatively common in patients with glioblastoma treated with bevacizumab. In the BRAIN trial, 27.4% of patients in the bevacizumab-alone arm, and 40.5% in the bevacizumab and irinotecan arm experienced grade 1 or 2 systemic hemorrhages.20 However, grade 3/4 hemorrhages were uncommon, occurring in <2% of patients in this study, and in 0%–4% of other trials of bevacizumab in patients with glioblastomas.22,24,55,56 Similarly, the rate of grade 3/4 hemorrhages in patients with glioblastoma treated with VEGFR inhibitors is low: 3% with cabozantinib (XL184)57 and <1% with cediranib.58 In summary, although for years, bevacizumab was not available for treating patients with glioblastoma because of concerns about the potential risk of intracerebral hemorrhage, all the studies to date have shown that the risk of intracerebral hemorrhage is low, ranging from 1% to 3.8%.20,21,59,60
There is also increasing evidence that patients with brain metastases treated with bevacizumab have a low risk of intracerebral hemorrhage. None of the 115 patients in the AVF3752g (PASSPORT) trial in which patients with non small-cell lung cancer and brain metastases treated with bevacizumab had grade ≥2 intracerebral hemorrhage.61 In a retrospective analysis from 13 randomized controlled trials of bevacizumab, compiling 187 patients with occult brain metastases, 3.3% of patients with brain metastases had grade 4 cerebral hemorrhage, compared with 1 grade 5 hemorrhage (1%) in control patients. In the same report, 3 (0.9%) of 321 patients with occult metastases in single-arm studies who received bevacizumab developed cerebral hemorrhage, and 1 (0.8%) of 131 patients with brain metastases treated without bevacizumab developed a grade 2 cerebral hemorrhage.62
These data suggest that the risk of intracerebral hemorrhage in patients with brain tumor treated with anti-VEGF/VEGFR therapy is low. In the absence of overt intratumoral hemorrhage, treatment of primary and secondary brain tumors with anti-VEGF/VEGFR therapies is relatively safe. Exceptions may include those metastases with a higher propensity to hemorrhage, such as melanoma and choriocarcinoma.
Thrombosis and Other Vascular Events
Venous thromboembolic events (VTE) are common in patients with malignant gliomas. A review of published studies examining the risk of VTE in patients with malignant glioma reported that the 6-week peri-operative period had the highest rate of VTE.63 However, after this initial period, the overall risk of VTE (17 months of follow up) is 24%. More recently, a retrospective review of the incidence of VTE in patients with malignant glioma compiled data on 9489 patients.52 This large study revealed an overall incidence of VTE of 7.5%, with more than half of the events occurring within 2 months of a neurosurgical procedure. This study also identified risk factors for developing VTE, including age >65 years, diagnosis of glioblastoma, and recent neurosurgical procedures. A comparable increase in the risk of arterial thrombosis has not been reported for patients with malignant gliomas.
Vascular events, including venous and arterial thrombosis, have been reported as a frequent complication of anti-angiogenic therapies. However, the variable, often high reported rate of VTE in patients with malignant gliomas makes it difficult to determine whether the reported incidence of VTE exceeds the anticipated rate associated with the disease. Conversely, because arterial thromboses are uncommon in patients with malignant gliomas, arterial events are more likely to be considered directly related to anti-angiogenic therapy.
Early anti-angiogenic agents, notably thalidomide, were associated with a marked increase in the incidence of both venous and arterial thromboses.65,66 For many of the systemic cancers treated with chemotherapy combinations with thalidomide, the increase in the incidence of thromboembolic events was dramatic. In a study of multiple myeloma, venous thrombotic events were noted in 34%, and in a separate study, a chemotherapy combination regimen including thalidomide for renal cell cancer had a VTE rate of 43%.67 In addition, arterial thrombosis was reported in 7% of patients with prostate cancer treated with thalidomide.68 These findings have led to the recommendation that thalidomide be administered in conjunction with low-dose warfarin, although the protective impact of a low-dose anticoagulant has not been fully established. Thalidomide has been used as a treatment for patients with malignant gliomas. In reported studies that did not include warfarin prophylaxis, the rate of VTE was ∼20%.69,70 Similar rates of VTE have been reported with other immunomodulatory agents, such as lenalidomide, that are currently under investigation for a variety of primary brain tumors, including glioblastoma and pediatric infiltrative brainstem gliomas.71 These findings underscore the potential for anti-angiogenic therapies to augment already high rates of VTE in patients with brain tumor.
The introduction of more-targeted anti-angiogenic agents, such as bevacizumab, generated concerns regarding treatment-induced arterial and venous thrombosis. Early phase II studies of bevacizumab in a wide variety of cancers reported rates of venous and arterial thrombosis ranging from 3% to 19% but did not include patients with glioma because of concerns about tumor and brain hemorrhage. An initial analysis that compiled data from 5 randomized (non-CNS tumor) trials of chemotherapy with or without bevacizumab that comprised 1745 patients determined that there was no increased risk of VTE with the addition of bevacizumab (P = .44).52 However, a subsequent meta-analysis compiling the results of 15 randomized controlled trials reported a significantly increased risk of VTE in patients treated with bevacizumab.72 In this study evaluating a total of 7956 patients, the RR of developing VTE was 1.33 with use of bevacizumab (P < .001). Furthermore, the risk was unchanged with the use of low-dose bevacizumab (2.5 mg/kg/week), compared with high dose (5.0 mg/kg/week).
All studies, to date, of bevacizumab in recurrent malignant gliomas have been relatively small, and none has included a treatment arm without bevacizumab to allow comparison of the RR of VTE with and without the drug. The incidence of VTE in these studies has ranged from 5% to 10% (Table 2). The combination of signal transduction modulator erlotinib with bevacizumab did not signficiantly increase the risk of VTE.73 In addition, cediranib, an oral TKI that directly targets the VEGF receptor, was tested in patients with recurrent glioblastoma and produced only a low incidence of VTE.58
We obtained unpublished data from Genentech from the Avastin Adverse Event database74 for both venous (VTE) and arterial (ATE) thrombotic events in patients undergoing treatment with a bevacizumab-containing regimen to provide a foundation to compare incidence in other solid tumors with high-grade gliomas. The database defined a VTE as a deep venous thrombosis, pulmonary embolus, mesenteric venous occlusion, retinal vein thrombosis, upper extremity thrombosis, or phlebitis. A small percentage of the patients in this database were in studies in glioblastoma, and these data reveal that the overall incidence of VTE (on study) was 7%. However, patients receiving bevacizumab alone had an incidence of 3.6%, compared with 8.9% with the combination of bevacizumab and irinotecan. For colorectal cancer, the rate of grade 3 or 4 VTE was 15% among patients treated with bevacizumab with chemotherapy, compared with 13.6% when treatment was chemotherapy alone. Similarly, in non small-cell lung cancer, the incidence of VTE was 5.6% with bevacizumab, compared with 3.2% with chemotherapy alone. Of interest, in breast cancer, the incidence of VTE was 3% with bevacizumab and paclitaxel and 4.3% with placebo and paclitaxel. Overall, the only risk factor for developing VTE was the addition of bevacizumab to chemotherapy.
The database defined an ATE as angina, stroke, myocardial infarction, transient ischemic attack, atrial fibrillation, or peripheral vascular disease. Overall, an evaluation of randomized controlled trials revealed that the incidence of grade 3 or higher ATEs was 2.4% with bevacizumab-containing regimens, compared with 0.7% with chemotherapy regimens without bevacizumab (data obtained from the Genentech Adverse Event Database). Similarly, pooling data from 5 studies in non small-cell lung cancer, breast, and colorectal cancer that evaluated bevacizumab-containing regimens (1745 patients) revealed an incidence of ATE of 3.8%. Risk factors for developing ATE from the entire database included age >75 years, history of arterial disease, baseline hypertension, an Eastern Cooperative Oncology Group (ECOG) performance status >2, and nonreceipt of anticoagulation therapy while undergoing treatment with bevacizumab. The BRAIN study, which treated 163 patients with recurrent glioblastoma with either bevacizumab alone or in combination with irinotecan, reported grade 3–5 ATEs in 3% of patients, consistent with other solid tumor studies.20
Assessment and management of thrombotic complications is complex and requires assessment of the impact of the anti-angiogenic agent on the event and the risk of treating the thrombosis. Most clinical trials that incorporate anti-angiogenic treatments permit continuing the therapy when there is evidence that the VTE is resolving. The recommended treatment for the VTE includes the use of standard anticoagulants and the continuation of bevacizumab in the absence of hemorrhage. Although there are no direct comparisons of the use of low–molecular weight heparin with oral warfarin in this context, there is a trend toward the use of low–molecular weight heparin because of reports of improved efficacy and no issues of drug-drug interactions or impact of diet on efficacy that are prominent with warfarin.75
The risk of anticoagulation in patients with glioma treated with bevacizumab has been investigated. A small series with 21 patients looked at concomitant anticoagulation with bevacizumab treatment, both given for a mean of 72 days.76 There were no lobar hemorrhages; 3 small parenchymal hemorrhages were detected, with 1 being symptomatic and the others petechial. A larger study retrospectively reviewed 282 patients with high-grade glioma treated with bevacizumab, with 64 patients also receiving a systemic anticoagulant. Overall, the hemorrhage rate in the group receiving anticoagulation was 20%, with intracerebral hemorrhage accounting for half of the events, 2 (3%) of which were grade 4. Most of the intracerebral hemorrhages reported were asymptomatic and detected as punctuate changes on MRI. Two patients (1%) who were treated with bevacizumab but not anticoagulation had serious intracerebral hemorrhage.
Patients who develop ATE should have the anti-angiogenic treatment stopped. Treatment of an ATE should be guided by the disease process, recognizing that the optimal management of stroke, myocardial infarction, and peripheral vascular occlusion may be quite different. No guidelines currently exist for the nonclassic events, such as the chronic diffusion restriction and/or apparent diffusion coefficient (ADC) decrease changes that have been reported with bevacizumab, as described above. The pathogenesis and clinical importance of these findings have not yet been well defined, although ongoing research may soon provide some guidance.
The identification of stroke in patients undergoing anti-angiogenic therapy may be challenging. MRI, particularly diffusion-weighted imaging (DWI) and ADC mapping are well established as diagnostic tools for conventional strokes. Typically, changes on DWI and ADC occur acutely with the event and resolve within a few weeks. However, vascular events that develop with anti-angiogenic agents may not follow this pattern (see Fig. 2). A recent study reported on 18 consecutive patients who were undergoing treatment with bevacizumab and were prospectively evaluated with DWI and ADC imaging.77 Thirteen of the 18 patients developed stroke-like lesions, defined as diffusion restriction on DWI with an accompanying decrease in ADC. Most of these changes were not associated with clinical findings. Of interest, these imaging abnormalities lasted up to 80 weeks.
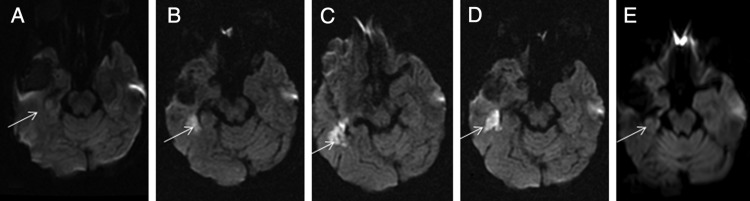
Fig. 2.
Imaging changes associated with a vascular event in a patient treated with bevacizumab. A, Pretreatment. B, Ten months later. Rapid onset of weakness. Bevacizumab stopped. C,Twelve months later, 2 months after stopping bevacizumab. D, Fifteen months, 5 months after stopping bevacizumab. E, Twenty-one months after stopping bevacizumab.
Wound Healing
Angiogenesis is a necessary step in wound healing. Anti-angiogenic agents potentially interfere with wound healing by impairing neovascularization, disturbing platelet-endothelial cell interaction, and reducing VEGF-induced tissue factor on endothelial cells.78 The long half-life of bevacizumab of 20 days (range, 11–50 days) results in a more extended risk of wound healing, compared with VEGFR inhibitors, which have a shorter half-life and usually less wound healing issues. In one study of patients with colorectal cancer who underwent surgery, 10 (13%) of 75 patients who had surgery within 60 days of bevacizumab treatment had complications, compared with complications in only 1 (3.4%) of 29 patients who had surgery after chemotherapy alone.78
The frequency of wound breakdown of all grades in patients with glioblastoma treated with bevacizumab ranges from 0% to 6%.20,22,24,55,79 Similarly low rates have been reported with VEGFR inhibitors, such as cabozantinib (XL184; 2%).57
The impact of prior bevacizumab chemotherapy on craniotomy wound healing was recently evaluated in a retrospective review [74]. Two hundred nine patients underwent a repeat craniotomy, of whom 23 received preoperative bevacizumab and 18 received postoperative bevacizumab. Significantly more patients receiving preoperative bevacizumab developed healing complications (35%) than nonbevacizumab-treated patients (10.0%; P = .004). Postoperative bevacizumab was associated with 6% impaired healing, which was not significantly different from nonbevacizumab-treated control subjects (P >.99). The wound healing complications were more striking for the third craniotomy than for the second craniotomy and for a shorter delay between bevacizumab and surgery. On the basis of these results, the authors recommended performing repeated craniotomy after a minimum of 28 days after last administered dose of bevacizumab whenever possible.80
Because many patients receiving bevacizumab require placement of venous access ports, the effect of the agent on wound dehiscence or impaired wound healing is a common clinical issue. In one study involving 195 ports placed in 189 patients, the incidence of wound dehiscence was significantly higher in those patients receiving bevacizumab within 10 days of port placement.81 A more recent retrospective review evaluated wound healing in 1108 port placements in patients who were treated with bevacizumab.78 Patients treated with bevacizumab within 1 day of port placement had an absolute risk of wound dehiscence requiring chest wall port explant of 2.4%. The risk of wound dehiscence was inversely proportional to the interval between bevacizumab administration and port placement, with significantly higher risk seen when the interval was <14 days.82
Although studies are limited, ideally, bevacizumab should be avoided 4 weeks before and after surgery. For smaller surgeries, bevacizumab should be avoided for at least 2 weeks, whenever possible. Surgery can usually be performed sooner with VEGFR inhibitors because of their shorter half-life, but a washout of at least 1 week is recommended.
Bowel Perforation
Anti-angiogenic agents can contribute to bowel perforation by several mechanisms, including tumor necrosis, exacerbation of existing gastric ulcers or diverticulitis, obstruction, chemotherapy-associated colitis, ischemic perforation of normal bowel or anastomosis, arterial thromboembolic events, and exacerbation of steroid effects.53 The risk factors most relevant to patients with brain tumor include underlying diverticular disease and peptic ulceration, constipation, and the concomitant use of corticosteroids.
In a meta-analysis compiling 12 294 patients with a variety of solid tumors from 17 randomized controlled trials, the incidence of gastrointestinal perforation was 0.9% (95% CI, 0.7%–1.2%). The RR was 2.14 (95% CI, 1.19–3.85; P = .011), and mortality was 21.7% (95% CI, 11.5%–37.0%). Most of the cases of gastrointestinal perforation occurred within the first 6 months of treatment.83 The RR was higher for those receiving 5 mg/kg of bevacizumab (2.67; 95% CI, 1.14–6.26) than for those receiving 2.5 mg/kg (1.61; 95% CI, 0.76–3.38). Higher risks were also observed among patients with colorectal carcinoma (RR, 3.10) and renal cell cancer (RR, 5.67).53
There are only a limited number of studies addressing the risk of bowel perforation in patients with brain tumor. In one retrospective review of 244 patients with high-grade glioma treated with anti-angiogenic agents, predominantly bevacizumab, 6 developed bowel perforation (2.5%); 2 of these patients died, and 4 eventually recovered.20 All of these patients had received concomitant corticosteroids.84 The incidence of bowel perforation in clinical trials of bevacizumab in patients with glioblastomas range from 0% to 2.9%.21,22,24,59,85 Fewer data are available for patients with glioblastomas treated with VEGFR inhibitors. In a trial involving 153 patients treated with cabozantinib (XL184), the incidence of bowel perforation was 2%.57
Bowel perforation is generally associated with a high mortality and requires prompt surgical assessment.86 A nonoperative approach involving bowel rest, intravenous fluids, and broad-spectrum antibiotics is usually preferred, although surgical intervention is sometimes necessary. However, this latter treatment is often complicated by problems with wound healing.
Conclusions
Bevacizumab is now routinely used in the treatment of patients with malignant glioma Other anti-angiogenic agents are being evaluated and may also be used in the treatment of malignant gliomas because of the importance of angiogenesis in tumor growth. Although angiogenesis is an enticing target for therapy, these agents have well-recognized complications. Common and significant toxicities include hypertension, proteinuria and risk for renal failure, posterior leukoencephalopathy syndrome, venous and arterial thromboembolic disease, bowel perforation, and poor wound healing. Diligent evaluation for these toxicities is important, because early intervention may decrease morbidity and mortality risk. Prompt recognition of an anti-angiogenic agent–related toxicity may also mandate treatment cessation to avoid exacerbation of the adverse event(s). Currently, the data on the occurrence and optimal management of these treatment-related complications in patients with gliomas are limited. Therefore, much of the available knowledge and management guidelines are based on data from the experiences in a variety of systemic cancers. Future studies that systematically evaluate potential clinical and genetic risk factors for toxicities, to determine the true incidence of these toxicities in the brain tumor population, leading to the establishment of both screening and treatment guidelines, are needed.
Acknowledgments
We thank Dr. Emaad Abdel-Rahman for his careful review.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Prados MD, Gutin PH, Phillips TL, et al. Highly anaplastic astrocytoma: a review of 357 patients treated between 1977 and 1989. Int J Radiat Oncol Biol Phys. 1992;23:3–8. doi: 10.1016/0360-3016(92)90537-r. [DOI] [PubMed] [Google Scholar]
- 3.Prados MD, Seiferheld W, Sandler HM, et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int J Radiat Oncol Biol Phys. 2004;58:1147–1152. doi: 10.1016/j.ijrobp.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 4.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 5.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Antiangiogenesis in cancer therapy–endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Sholley MM, Ferguson GP, Seibel HR, et al. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab Invest. 1984;51:624–634. [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 11.Bacher M, Schrader J, Thompson N, et al. Up-regulation of macrophage migration inhibitory factor gene and protein expression in glial tumor cells during hypoxic and hypoglycemic stress indicates a critical role for angiogenesis in glioblastoma multiforme. Am J Pathol. 2003;162:11–17. doi: 10.1016/S0002-9440(10)63793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasari VR, Kaur K, Velpula KK, et al. Downregulation of Focal Adhesion Kinase (FAK) by cord blood stem cells inhibits angiogenesis in glioblastoma. Aging (Albany NY) 2010;2:791–803. doi: 10.18632/aging.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong ET, Brem S. Taming glioblastoma by targeting angiogenesis: 3 years later. J Clin Oncol. 2011;29:124–126. doi: 10.1200/JCO.2010.32.5282. [DOI] [PubMed] [Google Scholar]
- 14.Junck L. Bevacizumab antiangiogenic therapy for glioblastoma. Neurology. 2002;76:414–415. doi: 10.1212/WNL.0b013e31820a0d7e. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 16.Chinot OL, de La Motte Rouge T, Moore N, et al. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28(4):334–340. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 17.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vredenburgh JJ, Desjardins A, Kirkpatrick JP, et al. Addition of Bevacizumab to Standard Radiation Therapy and Daily Temozolomide Is Associated with Minimal Toxicity in Newly Diagnosed Glioblastoma Multiforme. Int J Radiat Oncol Biol Phys. 2011;17(12):4119–4124. doi: 10.1016/j.ijrobp.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 19.Moen MD. Bevacizumab: in previously treated glioblastoma. Drugs. 2010;70:181–189. doi: 10.2165/11203890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 21.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71:1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 23.Schiff D, Purow B. Bevacizumab in combination with irinotecan for patients with recurrent glioblastoma multiforme. Nat Clin Pract Oncol. 2008;5:186–187. doi: 10.1038/ncponc1077. [DOI] [PubMed] [Google Scholar]
- 24.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhary S, Wong ET. Bevacizumab combined with irinotecan for recurrent glioblastoma multiforme–improvement over available therapy? Nat Clin Pract Neurol. 2008;4:242–243. doi: 10.1038/ncpneuro0712. [DOI] [PubMed] [Google Scholar]
- 26.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 27.Kamoun WS, Ley CD, Farrar CT, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27:2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med. 2009;122:322–328. doi: 10.1016/j.amjmed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Shord SS, Bressler LR, Tierney LA, et al. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm. 2009;66:999–1013. doi: 10.2146/ajhp080455. [DOI] [PubMed] [Google Scholar]
- 30.Mourad JJ, des Guetz G, Debbabi H, et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008;19:927–934. doi: 10.1093/annonc/mdm550. [DOI] [PubMed] [Google Scholar]
- 31.van der Veldt AA, de Boer MP, Boven E, et al. Reduction in skin microvascular density and changes in vessel morphology in patients treated with sunitinib. Anticancer Drugs. 2010;21:439–446. doi: 10.1097/CAD.0b013e3283359c79. [DOI] [PubMed] [Google Scholar]
- 32.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Wu S, Dahut WL, et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Ranpura V, Pulipati B, Chu D, et al. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens. 2010;23:460–468. doi: 10.1038/ajh.2010.25. [DOI] [PubMed] [Google Scholar]
- 35.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 36.Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 37.Gressett SM, Shah SR. Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother. 2009;43:490–501. doi: 10.1345/aph.1L426. [DOI] [PubMed] [Google Scholar]
- 38.Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izzedine H, Ederhy S, Goldwasser F, et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009;20:807–815. doi: 10.1093/annonc/mdn713. [DOI] [PubMed] [Google Scholar]
- 40.Yeh J, Frieze D, Martins R, et al. Clinical utility of routine proteinuria evaluation in treatment decisions of patients receiving bevacizumab for metastatic solid tumors. Ann Pharmacother. 2010;44:1010–1015. doi: 10.1345/aph.1M670. [DOI] [PubMed] [Google Scholar]
- 41.Izzedine H, Massard C, Spano JP, et al. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur J Cancer. 2010;46:439–448. doi: 10.1016/j.ejca.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bollee G, Patey N, Cazajous G, et al. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant. 2009;24:682–685. doi: 10.1093/ndt/gfn657. [DOI] [PubMed] [Google Scholar]
- 44.Overkleeft EN, Goldschmeding R, van Reekum F, et al. Nephrotic syndrome caused by the angiogenesis inhibitor sorafenib. Ann Oncol. 2010;21:184–185. doi: 10.1093/annonc/mdp472. [DOI] [PubMed] [Google Scholar]
- 45.McIntyre NJ, Taal MW. How to measure proteinuria? Curr Opin Nephrol Hypertens. 2008;17:600–603. doi: 10.1097/MNH.0b013e328313675c. [DOI] [PubMed] [Google Scholar]
- 46.Wu S, Kim C, Baer L, et al. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21:1381–1389. doi: 10.1681/ASN.2010020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lockhart AC, Rothenberg ML, Dupont J, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–214. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson ES, Matulonis UA, Ivy P, et al. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol. 2010;5:477–483. doi: 10.2215/CJN.08111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaughn C, Zhang L, Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr Oncol Rep. 2008;10:86–91. doi: 10.1007/s11912-008-0013-z. [DOI] [PubMed] [Google Scholar]
- 50.Cumurciuc R, Martinez-Almoyna L, Henry C, et al. Posterior reversible encephalopathy syndrome during sunitinib therapy. Rev Neurol (Paris) 2008;164:605–607. doi: 10.1016/j.neurol.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Lou E, Turner S, Sumrall A, et al. Bevacizumab-Induced Reversible Posterior Leukoencephalopathy Syndrome and Successful Retreatment in a Patient With Glioblastoma. J Clin Oncol. 2011;29:e739–e742. doi: 10.1200/JCO.2011.36.1865. [DOI] [PubMed] [Google Scholar]
- 52.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 53.Hapani S, Sher A, Chu D, et al. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79:27–38. doi: 10.1159/000314980. [DOI] [PubMed] [Google Scholar]
- 54.Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- 55.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2010;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilbert MR, Wang M, Aldape K, et al. A randomized phase II trial of bevacizumab with either Irinotecan (CPT) or Dose-Dense Temozolomide (TMZ) in recurrent Glioblastoma (GBM) Neuro-Oncology. 2010;11:iv36–iv57. doi: 10.1007/s11060-016-2288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen PY, Prados M, Schiff D, et al. Phase 2 study of XL184(BMS 907351), an inhibitor of MET, VEBFR2, and RET i patients with progressive glioblastoma. Journal of Clinical Oncology. 2010;28 suppl: abstr 2006. [Google Scholar]
- 58.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 60.Stark-Vance V. Bevacizumab and CPT-11 in the treatemnt of relapsed malignant glioma (abstract 342) Neuro-Oncology. 2005;7:369. [Google Scholar]
- 61.Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. 2009;27:5255–5261. doi: 10.1200/JCO.2009.22.0616. [DOI] [PubMed] [Google Scholar]
- 62.Besse B, Lasserre SF, Compton P, et al. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 2010;16:269–278. doi: 10.1158/1078-0432.CCR-09-2439. [DOI] [PubMed] [Google Scholar]
- 63.Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer. 2000;89:640–646. doi: 10.1002/1097-0142(20000801)89:3<640::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 64.Semrad TJ, O'Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg. 2007;106:601–608. doi: 10.3171/jns.2007.106.4.601. [DOI] [PubMed] [Google Scholar]
- 65.Zangari M, Tricot G, Polavaram L, et al. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high-dose dexamethasone. J Clin Oncol. 2010;28:132–135. doi: 10.1200/JCO.2009.23.0169. [DOI] [PubMed] [Google Scholar]
- 66.Zangari M, Fink LM, Elice F, et al. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol. 2009;27:4865–4873. doi: 10.1200/JCO.2009.22.3875. [DOI] [PubMed] [Google Scholar]
- 67.Desai AA, Vogelzang NJ, Rini BI, et al. A high rate of venous thromboembolism in a multi-institutional phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil and daily thalidomide in patients with metastatic renal cell carcinoma. Cancer. 2002;95:1629–1636. doi: 10.1002/cncr.10847. [DOI] [PubMed] [Google Scholar]
- 68.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2532–2539. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 69.Groves MD, Puduvalli VK, Chang SM, et al. A North American brain tumor consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81:271–277. doi: 10.1007/s11060-006-9225-y. [DOI] [PubMed] [Google Scholar]
- 70.Fadul CE, Kingman LS, Meyer LP, et al. A phase II study of thalidomide and irinotecan for treatment of glioblastoma multiforme. J Neurooncol. 2008;90:229–235. doi: 10.1007/s11060-008-9655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrier M, Le Gal G, Tay J, et al. Rates of Venous Thromboembolism in Multiple Myeloma Patients Undergoing Immunomodulatory Therapy with Thalidomide or Lenalidomide: A Systematic Review and Meta-Analysis. J Thromb Haemost. 2011;9(4):653–663. doi: 10.1111/j.1538-7836.2011.04215.x. [DOI] [PubMed] [Google Scholar]
- 72.Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 73.Sathornsumetee S, Desjardins A, Vredenburgh JJ, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010;12:1300–1310. doi: 10.1093/neuonc/noq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gentoutech, Inc. Avastin (bevacizumab) solution for intravenous infusion: US prescribing Information. South San Francisco (CA). September 2009. [Google Scholar]
- 75.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 76.Nghiemphu PL, Green RM, Pope WB, et al. Safety of anticoagulation use and bevacizumab in patients with glioma. Neuro Oncol. 2008;10:355–360. doi: 10.1215/15228517-2008-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rieger J, Bahr O, Muller K, et al. Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. J Neurooncol. 2010;99:49–56. doi: 10.1007/s11060-009-0098-8. [DOI] [PubMed] [Google Scholar]
- 78.Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 79.Raizer JJ, Grimm S, Chamberlain MC, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116:5297–5305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 80.Clark AJ, Butowski NA, Chang SM, et al. Impact of bevacizumab chemotherapy on craniotomy wound healing. J Neurosurg. 2011;114(6):1609–1616. doi: 10.3171/2010.10.JNS101042. [DOI] [PubMed] [Google Scholar]
- 81.Zawacki WJ, Walker TG, DeVasher E, et al. Wound dehiscence or failure to heal following venous access port placement in patients receiving bevacizumab therapy. J Vasc Interv Radiol. 2009;20:624–627. doi: 10.1016/j.jvir.2009.01.022. quiz 571. [DOI] [PubMed] [Google Scholar]
- 82.Erinjeri JP, Fong AJ, Kemeny NE, et al. Timing of administration of bevacizumab chemotherapy affects wound healing after chest wall port placement. Cancer. 2010;117:1296–1301. doi: 10.1002/cncr.25573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 84.Norden AD, Drappatz J, Muzikansky A, et al. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol. 2009;92:149–155. doi: 10.1007/s11060-008-9745-8. [DOI] [PubMed] [Google Scholar]
- 85.Kreisl TN, Zhang W, Odia Y, et al. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2010;13:1143–1150. doi: 10.1093/neuonc/nor091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Badgwell BD, Camp ER, Feig B, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol. 2008;19:577–582. doi: 10.1093/annonc/mdm508. [DOI] [PubMed] [Google Scholar]