Abstract
Cerebellar mutism syndrome (CMS) is an important medical challenge in the management of pediatric posterior fossa brain tumors, because it occurs in a subset of children following tumor resection. A definitive clinical profile and neuroanatomical substrate associated with CMS remains unclear. We investigated the relationship between presurgical and clinical variables and the incidence of CMS, along with diffusion tensor imaging, to characterize the integrity of cerebello-thalamo-cerebral white matter pathways. Seventeen children with posterior fossa tumors and CMS, 34 children with posterior fossa tumors without CMS, and 28 healthy children were enrolled in this study. Bilateral cerebello-thalamo-cerebral pathways were delineated and segmented into anatomical regions. Mean integrity measures for each region were compared among children with CMS, children without CMS, and healthy children. Left-handedness, medulloblastoma histology, and larger tumor size distinguished between patients with CMS and patients without CMS (P < .04). Right cerebellar white matter within the cerebello-thalamo-cerebral pathway was compromised in children with CMS relative to children without CMS and healthy children (P < .02). We provide a potential schema for CMS risk among children treated for posterior fossa tumors. Left-handed children treated for medulloblastoma may be the most at risk for CMS, and unilateral, localized damage within the cerebello-thalamo-cerebral pathway at the level of the right cerebellum is implicated in the presentation of CMS. This disruption in communication between the right cerebellum and left frontal cortex may contribute to speech-language problems observed in children with CMS. Our findings may be relevant for surgical planning and speech-language therapy to mitigate symptoms of CMS.
Keywords: cerebellar mutism syndrome, cerebello-thalamo-cerebral pathway, diffusion tensor imaging, posterior fossa syndrome, posterior fossa tumors
Cerebellar mutism syndrome (CMS), also known as posterior fossa syndrome (PFS), occurs in up to 25% of children following resection of pediatric posterior fossa (PF) tumors.1–3 CMS typically manifests 1–2 days postoperatively3 and is characterized by diminished speech output, dysarthria, and linguistic difficulties.1,4,5 Patients may also present with dysphagia, hypotonia, ataxia, emotional lability, and personality/behavioral changes.6 Children with CMS can have persistent, long-term neurological, speech-language, and cognitive impairment.3,7–9 Attempts to discern anatomical and clinical correlates of CMS have been made with variable results. Because CMS is a significant neurological complication following resection of pediatric PF tumors, understanding the relations between these risk factors and neuroanatomic insult is crucial. To date, no comprehensive model integrating clinical and neuroanatomic predictors has been developed. Such a model is essential for accurately predicting those patients who may present with CMS and subsequently take steps to mitigate the adverse effects of this syndrome. To develop our model, we examined clinical factors that may predict CMS, defined the primary white matter pathway from the cerebellum that has been previously implicated in CMS, and modeled the relations between these factors. CMS is a disorder of speech and language, which are dependent on hemispheric dominance. Thus, we also included premorbid handedness in our model to capture the influence of presurgical hemispheric organization on vulnerability to CMS.
First, there are numerous clinical factors that may contribute to CMS (see Wells et al.10 for a detailed summary). Previous studies have found no relation between CMS and age at diagnosis or sex.3,11,12 Tumor type and location have been identified as risk factors, with higher rates of CMS found in medulloblastoma relative to other tumor types,10 particularly when the tumor is situated in the vermis.1 Mixed results regarding the effect of tumor size identify this variable as a risk factor in some studies13,14 and inconsequential in predicting CMS in others.3,15 Because no well-defined clinical schema of CMS risk exists, our first goal was to investigate and produce a model of presurgical and clinical predictors of CMS.
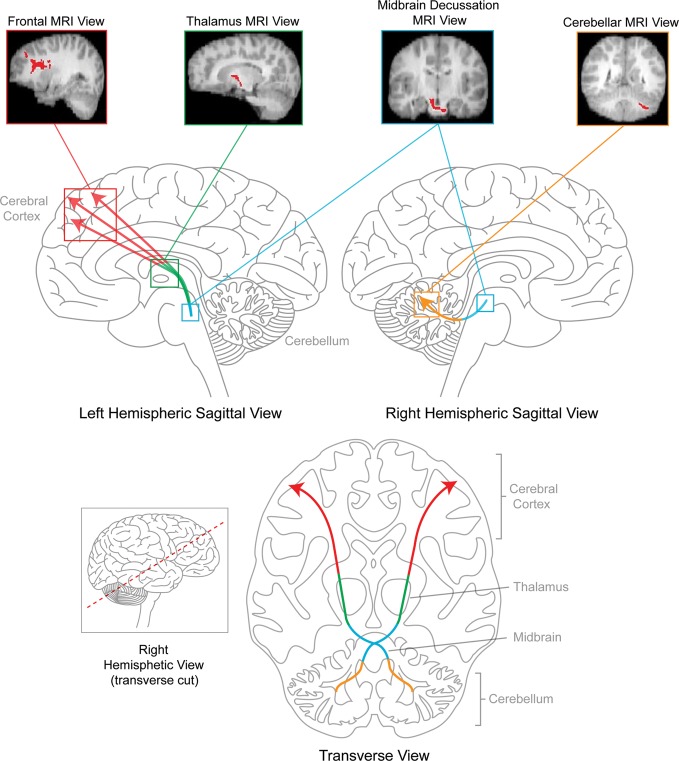
Second, attempts have been made to elucidate the mechanism of injury or neuroanatomical substrate that may explain why some patients present with CMS and others do not. An understanding of such a substrate is only now being uncovered. Increased brainstem involvement by the tumor and tumor infiltration of normal tissue have been associated with CMS.3 Furthermore, bilateral edema in the cerebellar peduncles following surgery has been implicated in CMS.16 The cerebellar peduncles connect the cerebellum with brainstem and are important components of cerebellar afferent/efferent pathways. Compromised cerebellar peduncles may contribute to the interruption in communication between the cerebellum and cortex, resulting in CMS. For example, bilateral hypoperfusion in proximal efferent cerebellar pathways (eg, cerebellar peduncles and frontal cortex) was observed in patients with CMS relative to patients without CMS.17 Such findings are consistent with a diaschisis model of pathophysiological insult in which injury in 1 brain region results in a loss of function in a distal brain region that is connected to the primary region of injury.17–21 Indeed, using a voxel-wise approach, Morris et al.22 found multiple white matter anomalies in patients with CMS, compared with patients without CMS across the brain, including areas connecting the cerebellum with the cortex. They did not, however, delineate and examine the integrity of the specific structural connections between the cerebellum and cortex via cerebellar peduncles. To define such connections and quantify the structural integrity of white matter pathways, we used diffusion tensor imaging (DTI) and probabilistic tractography. Our second goal was to determine whether CMS is related to structural damage in the cerebello-thalamo-cerebral (C-T-C) pathway with use of DTI methodologies (see Fig. 1).
Fig. 1.
A schematic of the C-T-C pathway. This bilateral C-T-C pathway originates in deep cerebellar nuclei, ascending through superior cerebellar peduncles, decussating in the rostral midbrain, and routing through ventrolateral thalamic nuclei into frontal cortex.
The C-T-C pathway is the primary efferent bilateral pathway connecting the cerebellum with the cortex.22–28 C-T-C pathways have been structurally documented using DTI tractography in healthy adults26 and children23 and in children with PF tumors.23 Because this pathway is a major cerebellar output circuit and has been shown to sustain damage following resection of PF tumors,23such damage may contribute to the symptoms of CMS.3,4,7,10,22,29,30 We hypothesized that patients with CMS would show greater structural compromise in the C-T-C pathway, particularly in the pons and cerebellar regions, than would patients without CMS or healthy control children.3,16,17
To develop our comprehensive model, we examined the relations between multiple presurgical and clinical variables and DTI measures of integrity of the C-T-C pathway in children with PF tumors. Comparisons were made between patients with CMS and those without CMS. Comparisons were also made with healthy control children for our DTI measures.
Materials and Methods
Participants
Seventeen children with PF tumors who presented with CMS following treatment, 34 children treated for PF tumors without CMS, and 28 healthy control children participated in this study. All patients were seen at the Hospital for Sick Children (SickKids), British Columbia Children's Hospital (BCCH), or Alberta Children's Hospital (ACH). Patients were excluded from the study if they were treated for recurrent disease, had diffuse brainstem glioma, were receiving palliative care, or had a premorbid history of neurological or learning disabilities. The protocol for this study was approved by the Research Ethics Boards of each participating site. All participants provided written informed consent/assent, and parental consent was obtained where applicable. Patients received a diagnosis of CMS if 2 criteria were met: (1) patients had undergone resection for PF tumor and (2) after the resection (typically 1–3 days after surgery), patients presented with markedly reduced speech output or no speech output on clinical examination. Presurgical handedness was assessed at diagnosis at neuropsychological evaluation. Tumor, treatment, and demographic variables were compared between the appropriate groups (see Table 1 for means and standard deviations). There were no differences among all groups for sex (χ2(2) = 2.104, P = .349) or age at time of DTI scan (F[2,76] = 0.185, P = .832). There were no age differences between children with CMS and children without CMS for handedness (F[1,41] = 0.131, P = .719) or tumor type (F[2,41] = 1.712, P = .193).
Table 1.
Demographic and medical variables for children treated for PF tumors with CMS following treatment, children treated for PF tumors without CMS, and healthy control children.
| CMS (n = 17) | No CMS (n = 34) | Controls (n = 28) | |
|---|---|---|---|
| Sex (% males) | 41.18 | 61.76 | 50.0 |
| Age at testing (years) | |||
| Mean (SD) | 11.27 (3.31) | 11.23 (3.79) | 10.76 (2.79) |
| Range | 7.17–17.08 | 5.33–17.33 | 5.75–17.17 |
| Handedness | |||
| Right | 11 (64.7%) | 33 (97.1%) | 25 (89.3%) |
| Left | 6 (35.3%) | 1 (2.9%) | 3 (10.7%) |
| Age at diagnosis (years) | |||
| Mean (SD) | 7.50 (1.91) | 7.98 (3.58) | N/A |
| Range | 4.89–12.75 | 1.40–15.66 | N/A |
| Time since diagnosis (years) | |||
| Mean (SD) | 3.54 (2.62) | 3.25 (3.01) | N/A |
| Range | 0.09–7.58 | 0.00–11.42 | N/A |
| Tumor Size (mm2)a | |||
| Mean (SD) | 2264 (722) | 1653 (924) | N/A |
| Range | 1224–3750 | 28–3300 | |
| Tumor Location within PF | |||
| Midline | 14 (82.35%) | 30 (88.24%) | N/A |
| Left Hemispheric | 0 | 3 (8.82%) | N/A |
| Right Hemispheric | 3 (17.65%) | 1 (2.94%) | N/A |
| Surgical outcome/extent of resection (%) | |||
| Greater than 95% of the tumor resected | 58.82 | 67.65 | N/A |
| Between 50% and 95% of the tumor | 23.53 | 17.65 | N/A |
| resected | |||
| Biopsy | 17.65 | 14.70 | N/A |
| Diagnosis | |||
| Low Grade Glioma/Astrocytoma | 2 (11.77%) | 13 (38.24%) | N/A |
| Ependymoma | 0 | 6 (17.64%) | N/A |
| Medulloblastoma | 14 (82.35%) | 13 (38.24%) | N/A |
| Germinoma | 0 | 1 (2.94%) | N/A |
| Choroid Plexus Papilloma | 0 | 1 (2.94%) | N/A |
| Ganglioglioma | 1 (5.88%) | 0 | N/A |
| Radiation Field and Dose (cGy)b | |||
| Craniospinal Radiation + PF Boost | n = 14 | n = 12 | N/A |
| Mean (SD) Head/Spine | 2700 (590.70) | 2715 (571.58) | N/A |
| Mean (SD) PF Boost | 3099 (675) | 2925 (1041) | N/A |
| Focal PF Radiation | n = 0 | n = 7 | N/A |
| Mean (SD) PF/Tumor | N/A | 5433 (376) | N/A |
| Chemotherapyc | |||
| Yes | 14 (82.35%) | 17 (50.00%) | N/A |
| No | 3 (17.65%) | 17 (50.00%) | N/A |
a Tumor size was calculated by multiplying the 2 largest measurements of the tumor from an anatomical MRI scan. Measurements are in mm2. Tumor size dimensions were not available for 9 patients.
b Note that the remainder of patients in each group were treated with surgery only and were not treated with radiation.
c Agents included Carboplatin, Cisplatin, Cyclophosphamide, Lomustine (CCNU), and Vincristine.
MR Imaging and Postprocessing
The details of our protocol have been described previously23 and are summarized here. MRI measurements were performed at SickKids using a GE LX 1.5T MRI scanner with an 8-channel head coil and at the BCCH and ACH using a Siemens 1.5T MRI scanner with a 12-channel head coil. The scanning protocol included a 3D-T1 FSPGR gradient echo, inversion recovery-prepared sequence (IR time = 400 ms; TE/TR = 4.2/10.056 ms; 116–124 contiguous axial slices; NEX = 1; 256 × 192 matrix, interpolated to 256 × 256; FOV = 24 × 24 cm; rbw = 162.734 kHz; slice thickness = 1.5 mm), and a diffusion-weighted sequence (single shot spin echo DTI sequence with EPI readout: 25–31 directions; b = 1000 s/mm2; TE/TR = 85.5/15 000 ms; 45–50 contiguous axial slices; NEX = 1; 128 × 128 matrix, interpolated to 256 × 256; FOV = 24 × 24 cm; rbw = 1953.12 kHz; slice thickness = 3 mm). Because MRI scanning parameters were different among the 3 hospitals (verified by differing signal-to-noise ratios), site of MRI scan was included as a covariate in all analyses of imaging data (see Law et al.23).
DTI generates quantitative indices that reflect white matter microstructure based on properties of water diffusion.31 These indices include fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). Lower measures of FA and higher measures of MD, AD, and RD are thought to reflect axonal degeneration and compromised myelin sheath integrity.32,33 DTI postprocessing, seed/waypoint placement, and probabilistic tractography were conducted using the FMRIB Software Library.34,35 MRI data were corrected for inhomogeneity and eddy current, and DTI indices maps were computed. The probability of connection among all voxels in each image was calculated and served as a basis for fiber tracking analyses.36 Standardized seed and waypoint masks were registered onto each individual's image with no diffusion weighting. Frontal white matter (eg, medial prefrontal cortex, inferior prefrontal gyrus and superior and middle frontal gyri) was used as the seed point, and the thalamus and cerebellar white matter were each used as waypoints for tractography to delineate the C-T-C pathway bilaterally. Frontal white matter was defined on the surface of the 3D T1 image and then registered into zero-diffusion weighted space and extended 2 mm into adjacent white matter. The thalamus was defined on the zero-diffusion weighted image as was hemispheric cerebellar white matter (using the cerebellar peduncles as the superior boundary and the pons as the inferior boundary). Tracts were thresholded and edited to eliminate erroneous streamlines. The resultant C-T-C pathways were parcellated into regions based on an anatomical template23,37,38 to examine regional integrity. Anatomical regions produced from this segmentation included bilateral frontal hemispheric white matter, internal capsule/thalamus, midbrain/pons, and cerebellar hemispheric white matter. Means and standard deviations for all DTI indices (FA, MD, AD, and RD) were calculated for each anatomical region.
Statistics
First, presurgical and clinical variables were examined between patients with and without CMS with use of univariate analyses of variance and χ2 tests. Second, a model predicting CMS from those variables found to be statistically significant from the above analyses was tested using logistic regression analyses. Third, we used multivariate analysis of variance, which served to control for multiple comparisons and collinearity in the DTI data, to determine differences among patients with CMS, patients without CMS, and healthy control children for C-T-C pathway structural integrity. Specifically, DTI indices of each anatomical region of the pathway were compared. As part of these omnibus analyses, planned tests of simple effects were used. This was done to examine the a priori hypothesis that patients with CMS show decreased white matter integrity in the C-T-C pathway relative to patients without CMS and healthy control children. Lastly, a model predicting C-T-C pathway integrity from presurgical and clinical variables was investigated using linear regression.
Results
Clinical Predictors of CMS
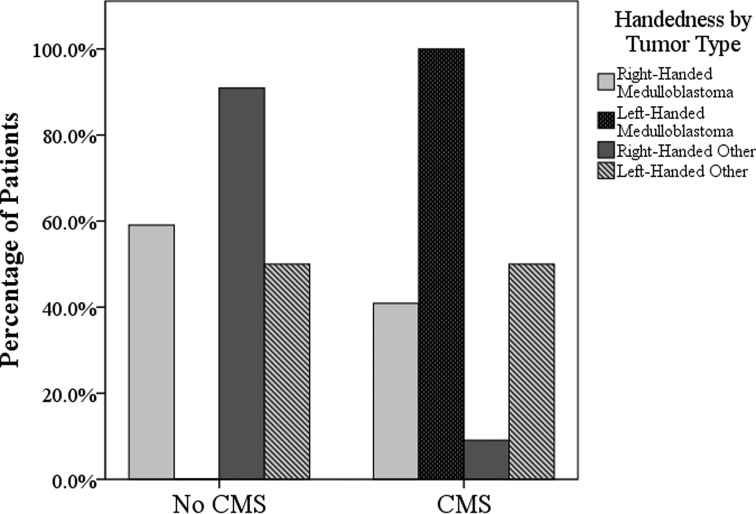
Children with CMS had larger tumor size (F[1,40] = 4.676, P = .037) than did children without CMS, and a greater proportion of children with CMS received a diagnosis of medulloblastoma (χ2(1) = 9.867, P = .02) (see Table 1). Furthermore, a greater proportion of children with CMS were left-handed (35%), compared with those without CMS (3%) (χ2(1) = 10.018, P = .004). Taking into consideration the known base rates for handedness (approximately 10% of the general population is left-handed),39 a disproportionate number of left-handed children presented with CMS (85%), compared with right-handed children (25%). In fact, in our sample, all left-handed patients with medulloblastoma presented with CMS (Fig. 2).
Fig. 2.
Presurgical and clinical predictors of CMS: the distribution of handedness and tumor pathology across patients with CMS and without CMS. Bars reflect within-group percentages. In terms of tumor pathology, “Other” signifies any other tumor type in our sample excluding medulloblastoma (eg, astrocytoma and ependymoma).
Age at diagnosis (F[1,49] = 0.266, P = .608), extent of tumor resection (χ2(1) = 0.399, P = .819), and tumor location (χ2(1) = 4.496, P = .106) did not differ between children with CMS and children without CMS (see Table 1). Although the majority of patients with CMS and without CMS had midline tumors (eg, vermis and fourth ventricle), 75% of those who had right cerebellar hemispheric tumors presented with CMS. Of the patients with left cerebellar hemispheric tumors, none presented with CMS.
We entered the 3 aforementioned predictor variables (handedness, tumor size, and tumor pathology) into a logistic regression model to determine the greatest predictor(s) of CMS. The omnibus model was significant (P = .006) and extracted only handedness as the strongest predictor of CMS (β = −2.71, P = .02). Considering that right-handedness is most prevalent in the general population, we then examined predictors of CMS in only the right-handed patients. No variables were extracted from this model, but tumor pathology and tumor size both approached statistical significance (P = .067 and P = .089, respectively).
Neuroanatomical Differences in CMS
Bilateral C-T-C pathways were delineated in all children (see Figs 1 and 3). A multivariate group effect was observed for DTI indices of the right cerebellar region of the C-T-C pathway connecting this area with left frontal cortex (Λ = 0.763, P = .015). Specific univariate analyses showed significant group differences in MD (F[2,72] = 6.737, P = .002), AD (F[2,72] = 4.814, P = .011), and RD (F[2,72] = 4.012, P = .022) (see Table 2 for means and standard deviations). Post-hoc analyses revealed that children with CMS had higher mean MD and AD (P < .01) in the right cerebellar region than did children without CMS and healthy control children. A significant difference in RD was also evident between children with CMS and healthy control children (P < .01); this effect approached significance between children with CMS and those without CMS (P = .051).There were no differences between children without CMS and healthy control children for the right cerebellar region. Furthermore, no differences were found between groups for DTI indices of the remaining regions of the C-T-C pathway connecting the right cerebellum with left frontal cortex. The groups did not differ on the DTI indices in the C-T-C pathway connecting the left cerebellar hemisphere with the right frontal cortex.
Fig. 3.
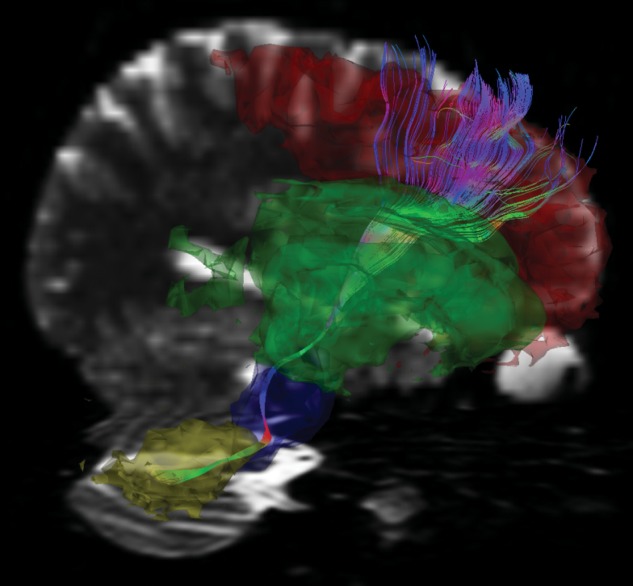
A sagittal view of the C-T-C pathway connecting right cerebellar hemispheric white matter with left frontal cortex via thalamus (in multicolor). The left frontal anatomical region of the C-T-C pathway falls within the red segmentation, the internal capsule/thalamus region of the pathway in the green segmentation, the midbrain/pons region of the pathway in the blue segmentation, and the cerebellar hemispheric white matter region of the pathway in yellow segmentation. Generated with MedINRIA.
Table 2.
Means and standard deviations (in parentheses) for regional DTI indices of the C-T-C pathway connecting the right cerebellar hemisphere with the left frontal cortex via the left thalamic nuclei for children with CMS, children without CMS, and healthy control children.
| C-T-C Pathway Anatomical Region | CMS |
No CMS |
Controls |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | MD (mm2/s) | AD (mm2/s) | RD (mm2/s) | FA | MD (mm2/s) | AD (mm2/s) | RD (mm2/s) | FA | MD (mm2/s) | AD (mm2/s) | RD(mm2/s) | |
| Left Frontal | 449 × 10−3 (81 × 10−3) | 768×10−6 (37 × 10−6) | 1171 × 10−6 (67 × 10−6) | 567 × 10−6 (68 × 10−6) | 425 × 10−3 (53 × 10−3) | 776 × 10−6 (38 × 10−6) | 1156 × 10−6 (67 × 10−6) | 578 × 10−6 (121 × 10−6) | 423 × 10−3 (52 × 10−3) | 777 × 10−6 (35 × 10−6) | 1154 × 10−6 (78 × 10−6) | 588 × 10−6 (44 × 10−6) |
| Left Thalamus/Internal Capsule | 515 × 10−3 (58 × 10−3) | 762 × 10−6 (45 × 10−6) | 1237 × 10−6 (64 × 10−6) | 524 × 10−6 (62 × 10−6) | 539 × 10−3 (67 × 10−3) | 746 × 10−6 (36 × 10−6) | 1251 × 10−6 (106 × 10−6) | 488 × 10−6 (120 × 10−6) | 509 × 10−3 (38 × 10−3) | 751 × 10−6 (31 × 10−6) | 1215 × 10−6 (63 × 10−6) | 519 × 10−6 (33 × 10−6) |
| Left Midbrain/Pons | 457 × 10−3 (56 × 10−3) | 903 × 10−6 (133 × 10−6) | 1346 × 10−6 (150 × 10−6) | 682 × 10−6 (132 × 10−6) | 491 × 10−3 (67 × 10−3) | 809 × 10−6 (105 × 10−6) | 1264 × 10−6 (169 × 10−6) | 571 × 10−6 (142 × 10−6) | 474 × 10−3 (26 × 10−3) | 846 × 10−6 (131 × 10−6) | 1285 × 10−6 (180 × 10−6) | 627 × 10−6 (109 × 10−6) |
| Right Cerebellum | 466 × 10−3 | 775 × 10−6 | 1191 × 10−6 | 567 × 10−6 | 461 × 10−3 | 712 × 10−6 | 1119 × 10−6 | 500 × 10−6 | 485 × 10−3 | 696 × 10−6 | 1103 × 10−6 | 492 × 10−6 |
| (63 × 10−3) | (101 × 10−6) | (135 × 10−6) | (99 × 10−6) | (110 × 10−3) | (56 × 10−6) | (89 × 10−6) | (128 × 10−6) | (55 × 10−3) | (61 × 10−6) | (90 × 10−6) | (60 × 10−6) | |
Note: Bolded cells indicate a significant mean difference between children with CMS and healthy control children at P < 0.01, while underlined numbers in cells indicates a significant mean difference between patients with CMS and patients without CMS at P < 0.01.
Our findings indicate that damage to the C-T-C pathway connecting the right cerebellum with left frontal cortex may underlie CMS. On the basis of the neuroanatomical difference that we found between patients with CMS and patients without CMS, other predictor variables may have an effect on neuroanatomical structure. Consequently, we examined whether the presurgical and clinical predictors that we found to be related with CMS were also associated with right cerebellar integrity of this pathway. Because MD of the right cerebellar region of the C-T-C pathway produced the strongest effect between patients with CMS and without CMS, tumor size, handedness, and tumor pathology were regressed on right cerebellar MD. This model was significant (F[1,37] = 12.108, P = .001), and again, only handedness was a significant predictor of right cerebellar white matter integrity within the C-T-C pathway (β = 0.497, P = .001).
Discussion
To our knowledge, this is the first study to include presurgical and clinical variables and neuroimaging to determine the risk factors of CMS following treatment for pediatric PF tumors. We observed a number of novel findings. On the basis of these findings, we have identified a neuroanatomical substrate associated with CMS and have laid the foundation for the development of an integrated model predicting those children most at risk for CMS following surgery.
A novel finding was that the majority of left-handed children in our sample presented with CMS following surgery. Furthermore, CMS was observed more frequently in patients with aggressive tumors (medulloblastoma) and patients with larger tumors. This is consistent with previous work showing that CMS occurs more often in patients who require a more radical resection of tumors.1 Of note, all children who were left-handed and treated for medulloblastoma presented with CMS postsurgically. There is a known vulnerability of left-handed children for neurological problems, including learning disabilities,40,41 autism,41 and epilepsy.41
Novel to the literature, we have demonstrated that compromise to the C-T-C pathway at the level of the cerebellum is implicated in CMS. Of importance, it is the unilateral damage to right cerebellar white matter that distinguished between patients who presented with CMS and those who did not. The specific insult seen within this pathway region is evidence that PF tumor resection may be associated with an interruption of cerebellocerebral communication. This disruption may contribute to the resultant problems in the coordination and processing of speech-language seen in CMS.
Lesions of the cerebellum can produce ataxia and dysarthria, particularly lesions to the right cerebellar hemisphere,42 and functionally, the cerebellum has been implicated in verb generation.43 Deficits in the initiation of language, verbal fluency, and word-finding ability have been found in children following PF tumor resection.7 Of interest, impairment in verbal intelligence and complex language tasks was observed in children who had undergone resection of cerebellar tumors and subsequently sustained damage to the right cerebellar hemisphere.4 Lesions of the right cerebellum may deprive left hemispheric cortical language areas of essential modulatory input, resulting in errors in language processing.42 Since the left frontal regions are important in mediating speech production and expressive language,44–48 our results provide evidence that the C-T-C pathway connecting the right cerebellum with areas of the brain important for speech production and expressive language (left frontal regions) is disrupted in CMS. It may be the axonal degeneration and loss of myelination within the right cerebellar region of the C-T-C pathway that is associated with CMS. We did not find group differences in any other pathway region. Because CMS is typically a postsurgical syndrome, it is logical that we would not initially expect compromise in any other region of the pathway. The deterioration of white matter pathways proximal to the cerebellum may take time to become evident; further damage in the pathway (eg, in pons and eventually thalamic and frontal cortex) would be expected on the basis of the extent of diaschisis (ie, progression to areas downstream of the initially damaged region at time of resection).
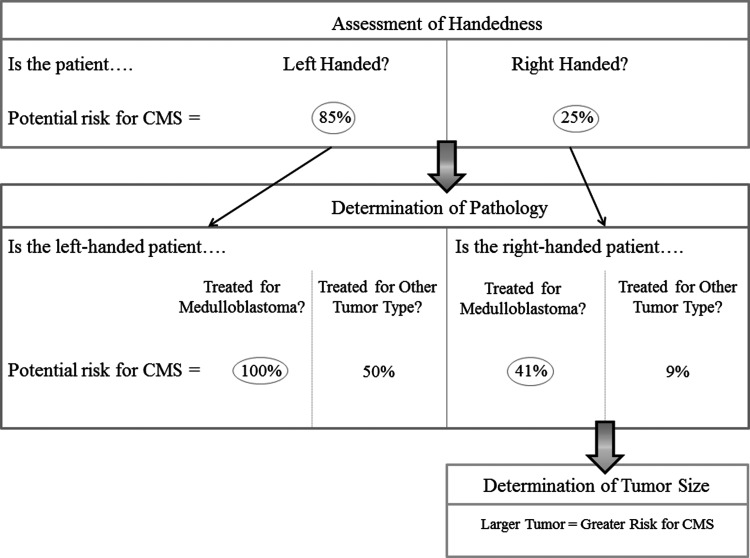
If we can identify patients who have a high risk of presenting with CMS following surgery, clinicians may be able to mitigate its symptoms. CMS may not be attributable solely to the effects of surgery, and presurgical variables may play a more prominent role in predicting CMS.49 Indeed, our results show that left-handedness predicts both CMS and C-T-C pathway white matter outcome following treatment for pediatric PF tumors. These parallel findings suggest that left-handed patients are at a greater risk for CMS following surgery and for increased vulnerability to C-T-C pathway white matter damage. In right-handed patients, it appears that tumor size, along with pathology, may be associated with CMS. In light of our findings, we have proposed a schema for determining the level of risk for CMS (Fig. 4). This model may have implications for clinically managing PF tumors and for lowering the risk for CMS. If compromise to the right cerebellar hemisphere is probable (eg, if the tumor is in the right hemisphere of PF or if this hemisphere will sustain damage caused by accessing the tumor), steps by surgeons may be warranted to ensure the preservation of healthy tissue in this region. Furthermore, if patients are left-handed and have medulloblastoma, more caution may be warranted for preserving as much right cerebellar hemispheric white matter as possible while still ensuring successful removal of tumor tissue. However, prospective validation of our findings is necessary to confirm that this proposed schema can be applied to the general population of children treated for brain tumors.
Fig. 4.
A potential schema for CMS risk, based on our quantitative and qualitative observations of presurgical and clinical variables. According to this schema left-handed patients are highly likely to present with CMS (85%); this chance rises to 100% if the left-handed patient is treated for medulloblastoma. Based on our sample, right-handed patients have a 25% chance of presenting with CMS in general; this chance becomes 41% if the right-handed patient is diagnosed with medulloblastoma. Further, larger tumor size is associated with greater risk for CMS in right-handed patients. Tumor size was not found to be a factor for CMS risk in our sample of left-handed patients. Under the determination of pathology section, “Other Tumor Type” includes ependymoma and low-grade glioma/astrocytoma, among others.
Our findings also have bearing on the implementation of preventative treatment plans for speech-language deficits following surgical intervention. Administering bromocriptine (a dopamine agonist) following the onset of CMS has shown some promise in mitigating akinetic mutism50,51 and has been shown to produce some (but inconsistent) resolution of cerebellar mutism. However, the mechanism through which these agents act on CMS is unknown and requires further research; knowledge gleaned from our present work is important in examining such mechanisms.
This study encompasses one of the largest samples for which both clinical and imaging data are available in the CMS literature. However, there are some limitations often seen in studies involving clinical samples. Because of the low base rate for left handedness in the population and the distribution of tumor pathology in our sample, cell sizes within these particular presurgical and clinical variables were relatively small. In addition, to avoid unnecessary delay in treating critically ill patients, DTI sequences are not typically included in presurgical MRI protocols in our institutions. Thus, there was a lack of preoperative DTI scans to compare C-T-C pathways before and after treatment (which is the reason that we used healthy control children as a comparison). Furthermore, because of the delay between surgery and neuroimaging for our study, we cannot distinguish between late DTI changes caused by functional alterations in CMS and DTI evidence of surgical injury (which may have caused CMS). It is nevertheless important to understand DTI white matter outcome measures in CMS, whether they are early or late, in our endeavor to predict a neuroanatomical correlate of this syndrome. In future studies, it will be important to examine C-T-C pathway integrity and the outcome and progression of CMS symptoms. Findings of this nature could also be correlated with changes in CMS symptomology to determine how, for example, mutism or behavioral problems are resolved in the weeks following treatment.
In summary, we have provided a clinical schema based on our quantitative and qualitative observations and have elucidated a neuroanatomical substrate that may contribute to the occurrence of CMS following treatment for PF tumors. We propose that left-handedness presurgically, larger tumor size, presence of higher grade tumor, and white matter damage in the right cerebellar region of the C-T-C pathway are associated with CMS. It will be important to prospectively test our model. Our findings contribute to the growing body of research on the predictors of CMS in patients with pediatric brain tumors. This knowledge may be of critical importance to oncological practice for surgical planning and implementation of preventative and/or mitigative measures to reduce speech-language morbidity and other symptoms associated with CMS.
Funding
This work was supported by the Pediatric Oncology Group of Ontario (to N. L.), C17 Research Network (to D. M.), and the Canadian Cancer Society (18062 to D. M.).
Conflict of interest statement. None declared.
References
- 1.Van Calenbergh F, Van de Laar A, Plets C, Goffin J, Casaer P. Transient cerebellar mutism after posterior fossa surgery in children. Neurosurgery. 1995;37(5):894–898. doi: 10.1227/00006123-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Pollack IF. Posterior fossa syndrome. Int Rev Neurobiol. 1997;41:411–432. doi: 10.1016/s0074-7742(08)60362-1. [DOI] [PubMed] [Google Scholar]
- 3.Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg. 2006;105(Suppl 6):444–451. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- 4.Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- 5.Vandeinse D, Hornyak JE. Linguistic and cognitive deficits associated with cerebellar mutism. Pediatr Rehabil. 1997;1(1):41–44. doi: 10.3109/17518429709060941. [DOI] [PubMed] [Google Scholar]
- 6.Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the Posterior Fossa Syndrome in children after cerebellar tumour surgery. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 2010;46(7):933–946. doi: 10.1016/j.cortex.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 8.Siffert J, Poussaint TY, Goumnerova LC, et al. Neurological dysfunction associated with postoperative cerebellar mutism. J Neurooncol. 2000;48(1):75–81. doi: 10.1023/a:1006483531811. [DOI] [PubMed] [Google Scholar]
- 9.Steinbok P, Cochrane DD, Perrin R, Price A. Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg. 2003;39(4):179–183. doi: 10.1159/000072468. [DOI] [PubMed] [Google Scholar]
- 10.Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev. 2008;14(3):221–228. doi: 10.1002/ddrr.25. [DOI] [PubMed] [Google Scholar]
- 11.Grill J, Viguier D, Kieffer V, et al. Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg. 2004;101(Suppl 2):152–158. doi: 10.3171/ped.2004.101.2.0152. [DOI] [PubMed] [Google Scholar]
- 12.Turgut M. Cerebellar mutism. J Neurosurg Pediatr. 2008;1(3):262. doi: 10.3171/PED/2008/1/3/262. [DOI] [PubMed] [Google Scholar]
- 13.Catsman-Berrevoets CE, Dongen HR, Van, Mulder PG, Paz y Geuze D, Paquier PF, Lequin MH. Tumour type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry. 1999;67(6):755–757. doi: 10.1136/jnnp.67.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelabert-Gonzalez M, Fernandez-Villa J. Mutism after posterior fossa surgery. Review of the literature. Clin Neurol Neurosurg. 2001;103(2):111–114. doi: 10.1016/s0303-8467(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 15.Wells EM, Khademian ZP, Walsh KS, et al. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr. 2010;5(4):329–334. doi: 10.3171/2009.11.PEDS09131. [DOI] [PubMed] [Google Scholar]
- 16.Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37(5):885–893. doi: 10.1227/00006123-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Miller NG, Reddick WE, Kocak M, et al. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol. 2010;31(2):288–294. doi: 10.3174/ajnr.A1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germano A, Baldari S, Caruso G, et al. Reversible cerebral perfusion alterations in children with transient mutism after posterior fossa surgery. Childs Nerv Syst. 1998;14(3):114–119. doi: 10.1007/s003810050191. [DOI] [PubMed] [Google Scholar]
- 19.Marien P, Engelborghs S, Michiels E, De Deyn PP. Cognitive and linguistic disturbances in the posterior fossa syndrome in children: a diaschisis phenomenon? Brain and Language. 2003;87(1):162–162. [Google Scholar]
- 20.Sagiuchi T, Ishii K, Aoki Y, et al. Bilateral crossed cerebello-cerebral diaschisis and mutism after surgery for cerebellar medulloblastoma. Ann Nucl Med. 2001;15(2):157–160. doi: 10.1007/BF02988609. [DOI] [PubMed] [Google Scholar]
- 21.Meyer JS, Obara K, Muramatsu K. Diaschisis. Neurol Res. 1993;15(6):362–366. doi: 10.1080/01616412.1993.11740164. [DOI] [PubMed] [Google Scholar]
- 22.Morris EB, Phillips NS, Laningham FH, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009;132(11):3087–3095. doi: 10.1093/brain/awp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law N, Bouffet E, Laughlin S, et al. Cerebello-thalamo-cerebral connections in pediatric brain tumor patients: impact on working memory. Neuroimage. 2011;56(4):2238–2248. doi: 10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 24.Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16(11):444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- 25.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salmi J, Pallesen KJ, Neuvonen T, et al. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22(11):2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- 27.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4(3):174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 29.Ozgur BM, Berberian J, Aryan HE, Meltzer HS, Levy ML. The pathophysiologic mechanism of cerebellar mutism. Surg Neurol. 2006;66(1):18–25. doi: 10.1016/j.surneu.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 31.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 32.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 33.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(Suppl 1):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 36.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabani NJ, Sled JG, Chertkow H. Magnetization transfer ratio in mild cognitive impairment and dementia of Alzheimer's type. Neuroimage. 2002;15(3):604–610. doi: 10.1006/nimg.2001.0992. [DOI] [PubMed] [Google Scholar]
- 38.Mabbott DJ, Rovet J, Noseworthy MD, Smith ML, Rockel C. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 39.Hardyck C, Petrinovich LF. Left-handedness. Psychological bulletin. 1977;84(3):385–404. [PubMed] [Google Scholar]
- 40.Geschwind N, Behan P. Left-handedness: association with immune disease, migraine, and developmental learning disorder. Proc Natl Acad Sci USA. 1982;79(16):5097–5100. doi: 10.1073/pnas.79.16.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewin J, Kohen D, Mathew G. Handedness in mental handicap: investigation into populations of Down's syndrome, epilepsy and autism. Br J Psychiatry. 1993;163:674–676. doi: 10.1192/bjp.163.5.674. [DOI] [PubMed] [Google Scholar]
- 42.Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115(1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 44.Broca P. Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech) Bulletin de la Societe Anatomique. 1861;6:330–357. [Google Scholar]
- 45.Geschwind N. Current concepts: aphasia. N Engl J Med. 1971;284(12):654–656. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- 46.Knecht S, Deppe M, Drager B, et al. Language lateralization in healthy right-handers. Brain. 2000;123(1):74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- 47.Knecht S, Drager B, Deppe M, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 48.Mayeux R, Kandel ER. Disorders of language: the aphasias. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principle of Neural Science. 3rd ed. London: Prentice Hall; 1991. pp. 839–851. [Google Scholar]
- 49.Di Rocco C, Chieffo D, Frassanito P, Caldarelli M, Massimi L, Tamburrini G. Heralding cerebellar mutism: evidence for pre-surgical language impairment as primary risk factor in posterior fossa surgery. Cerebellum. 2011;10(3):551–562. doi: 10.1007/s12311-011-0273-2. [DOI] [PubMed] [Google Scholar]
- 50.Caner H, Altinors N, Benli S, Calisaneller T, Albayrak A. Akinetic mutism after fourth ventricle choroid plexus papilloma: treatment with a dopamine agonist. Surg Neurol. 1999;51(2):181–184. doi: 10.1016/s0090-3019(98)00120-7. [DOI] [PubMed] [Google Scholar]
- 51.Catsman-Berrevoets CE, van Dongen HR, Zwetsloot CP. Transient loss of speech followed by dysarthria after removal of posterior fossa tumour. Dev Med Child Neurol. 1992;34(12):1102–1109. doi: 10.1111/j.1469-8749.1992.tb11424.x. [DOI] [PubMed] [Google Scholar]