Abstract
The Hirntumorstudien (HIT)-LGG-1996 protocol offered a comprehensive treatment strategy for pediatric patients with low-grade glioma (LGG), ie, observation, surgery, adjuvant radiotherapy, and chemotherapy to defer the start of irradiation in young children. In this current study, we sought to determine clinical factors for progression and survival. Between October 1, 1996 and March 31, 2004, 1031 patients were prospectively recruited into an observation arm (n = 668) and a nonsurgical arm stratifying 12 months of vincristine-carboplatin chemotherapy (n = 216) and conventional radiotherapy/brachytherapy (n = 147) in an age-dependent manner. Median patient age was 6.9 years; 28 patients had diencephalic syndrome, 44 had dissemination, and 108 had neurofibromatosis type 1(NF-1). Main tumor location was the supratentorial midline (40.4%), and the main histology was pilocytic astrocytoma (67.9%). Following a median observation of 9.3 years, 10-year overall survival (OS) was 0.94 and 10-year event-free survival (EFS) was 0.47. Ten-year progression-free survival was 0.62 following radiotherapy and 0.44 following chemotherapy. Sixty-one of 216 chemotherapy patients received radiotherapy 0.3–8.7 years after initial diagnosis. By multivariate analysis, diencephalic syndrome and incomplete resection were found to be unfavorable factors for OS and EFS, age ≥11 years for OS, and supratentorial midline location for EFS. Dissemination, age <1 year, and nonpilocytic histology were unfavorable factors for progression following radiotherapy (138 patients); and diencephalic syndrome, dissemination, and age ≥11 years were unfavorable factors following chemotherapy (210 patients). NF-1 patients and boys experienced prolonged tumor stabilization with chemotherapy. A nationwide multimodal treatment strategy is feasible for pediatric LGG. Extended follow-up yielded results comparable to single-institution series for the treatment groups. Three-quarters of surviving chemotherapy patients have not yet received radiation therapy. Infants with or without diencephalic syndrome and dissemination bear the highest risk for death and progression following diagnosis or treatment.
Keywords: chemotherapy, extended follow-up, low-grade glioma, radiotherapy
Low-grade gliomas (LGGs) of childhood and adolescence are the most common pediatric brain tumors and represent a spectrum of diseases. The effects of anatomic location, histology, underlying genetics, and age upon natural history and treatment are not yet fully understood.
A variety of medical disciplines contribute to the care of these children, and each discipline favors its main treatment approach. Surgical resection is considered the treatment of choice for most LGGs.1–8 A relevant proportion of tumors are not amenable to complete resection due to anatomic location or metastatic disease. Preceding our study, multiple publications reported the efficacy of radiotherapy and chemotherapy for selected groups of pediatric LGG cases of unresectable tumors.9–15 Indications for therapy differed and treatment algorithms were based on retrospective studies with limited patient numbers.16–19 Survival of pediatric LGG patients was high in these smaller series, but the significance of risk factor analysis for death or progression remained restricted.
The German multicenter, cooperative Hirntumorstudien (HIT)-LGG-1996 study was the first nationwide comprehensive treatment strategy for diagnosis, decision making, and therapy for children and adolescents with LGG with a framework of requirements mandatory for all patients. It had no pilot study. It included an observation arm as well as options for contemporary radiotherapy and chemotherapy. We aimed to prospectively study the feasibility of such an approach, as well as the efficacy of treatment with radiotherapy or chemotherapy, and to identify clinical risk factors for death and progression. The primary function of the chemotherapy arm was to defer the start of irradiation in young children.
To our knowledge, we here report the largest population-based and prospectively followed cohort with extended follow-up, comprising children and adolescents with LGG of all histologies and locations, testing the acceptability of a multidisciplinary treatment strategy.
Patients and Methods
Eligibility
Eligibility criteria included patient age <17 years with or without a clinical diagnosis of neurofibromatosis type 1 (NF-1) or other phakomatoses. Eligible diagnoses included LGG grade I or II according to World Health Organization (WHO)20,21 criteria at all CNS sites. Radiologic diagnosis was accepted for hypothalamic-chiasmatic tumors associated with NF-1 or for tumors extending along visual pathways (VPs) and demonstrating hypodensity on CT without application of contrast medium. MRI with defined sequences was requested as the appropriate modality for neuroimaging. Initial staging for cerebral primary tumors included spinal investigation in case of symptoms. If signs of seeding in the upper spinal area (upper cervical cord) were detected, whole-spine imaging was indicated.
Patients either had newly diagnosed disease during the time of the active study or had had their disease identified and observed prior to October 1, 1996, but had not received nonsurgical therapy and entered the treatment arms of the trial at the time of progression.
Informed consent was obtained from parents or guardians. Children or adolescents gave consent for themselves if they had appropriate understanding.
During the study period, no other trial for LGG was active in German-speaking countries. The German Speaking Society of Pediatric Oncology and Hematology (GPOH) approved the study. Central ethical approval was obtained, and the study was assigned the Gütesiegel A (Certificate of Quality) of the German Cancer Society, which includes a review for good clinical practice guidelines. Institutional review and approval followed local guidelines at the participating institutions.
Study Strategy
At diagnosis, best safe resection of the primary tumor was recommended. Patients with completely resected tumors were to be observed. Similarly, following incomplete resection/biopsy or radiologic diagnosis, an observation-only strategy was scheduled for children without threatening symptoms. Adjuvant radiotherapy or chemotherapy (“nonsurgical treatment”) was offered in case of severe or progressive symptoms or radiologic progression. A chemotherapy arm was instituted with the aim of deferring or even avoiding radiotherapy in young patients. This treatment arm was initially shared with the UK and Italian national study groups22 but continued until the end of the HIT-LGG-1996 recruitment period. At the beginning of the trial, it was planned that only children <5 years of age were to receive chemotherapy. However, during the study period a growing number of older children were primarily treated with chemotherapy, although irradiation was recommended as standard treatment in this age group. Regular clinical and radiologic assessments were scheduled.
Chemotherapy
Chemotherapy consisted of a 10-week induction phase with intravenous vincristine (1.5 mg/m², maximum 2 mg) given on day 1 of weeks 1–10 and single-dose carboplatin (550 mg/m²) as intravenous 1-hr infusions given on day 1 of weeks 1, 4, 7, and 10. During consolidation (wk 13–53), both drugs were given concomitantly every 4 weeks.23
Drug dosing was modified for children with body weight <10 kg (calculation per kg). Further reductions of one-third were recommended for children <6 months of age. Dose reductions were prescribed in cases of hematologic or organ toxicities. In case of an allergy to carboplatin, individual strategies were recommended depending upon the time point and severity of manifestation and the condition of the patient.
Radiotherapy
Local radiotherapy of the primary tumor region included safety margins of 1.0 cm (MRI based) or 2.0 cm (CT based), and ≤54 Gy and ≤50.4 Gy were given to cerebral and spinal tumors, respectively, in 1.8-Gy fractions following computer-assisted 3-D planning. In the event that irradiation of younger (<5 y) children was unavoidable, total doses of 40–45.2Gy were assigned in fractions of 1.6 Gy. Patients of any age with tumors suitable for brachytherapy (well-delineated small tumors at all sites) were treated with this modality.24,25
Salvage treatment
In case of progression during or after first nonsurgical therapy, salvage treatment was recommended with the hitherto “other” modality; that is, children who progressed after having had primary chemotherapy received radiotherapy as second-line treatment, and children who progressed after radiotherapy then received chemotherapy.
Methods
All patients with an LGG diagnosed between October 1, 1996, and March 31, 2004, were included in this report, along with their follow-up data until June 2011. Data concerning patient age, sex, NF-1 status, tumor location, extent of resection, and treatment, as well as follow-up information, were reviewed and verified at the trial center. Central pathologic review was strongly recommended and performed at the German Reference Center for Brain Tumors, Institute of Neuropathology, University of Bonn. Central radiologic review was offered during the latter part of the trial and performed at the Reference Center for Neuroradiology of the GPOH. Review of radiotherapy was offered for individual patients.
The distributions of overall survival (OS), event-free survival (EFS), and progression-free survival (PFS) were calculated according to the Kaplan–Meier method.26,27 As defined in this report, OS was calculated from date of diagnosis until death of the patient from any cause, and EFS was calculated from date of diagnosis until an “event,” ie, relapse, progression, the necessity to start nonsurgical treatment, or death from any cause. To evaluate the variables of resection and histology, OS and EFS were defined from date of resection until death/event or until last follow-up if no event occurred. PFS following nonsurgical treatment (either chemotherapy or radiotherapy) was calculated from start of therapy until an event, defined as progression of residual tumor, relapse following previous complete remission, appearance of new or progression of existing metastases, or death from any cause. To evaluate the variable response after week 24, PFS was defined from date of response until event or until last follow-up if no event occurred.
No survival analyses were performed for the observation group, since membership in the observation group changed over time—that is, each patient started as a member of the observation group and switched potentially to the chemotherapy or radiotherapy group—and Kaplan–Meier curves were applicable to only groups that did not change and were consistent over time.
Cox regression models with forward stepwise selection (inclusion criterion: score test P ≤ .05; exclusion criterion: likelihood ratio test P ≥ .10) were used to analyze the prognostic and predictive values of clinical and biologic variables on OS, EFS, and PFS. Such selection of variables has well-known advantages and disadvantages.28 Nevertheless, these methods are well accepted in exploratory analyses.
Variables included age (y) at diagnosis (both continuous and categorical: <1, 1–4, 5–10, and ≥ 11), sex, NF-1 status, presence of diencephalic syndrome (DS), dissemination, tumor location (hypothalamic-chiasmatic/vs other diencephalic/mesencephalic vs other; diencephalic vs all other; diencephalic vs brainstem vs spinal vs other), extent of resection, and histology (pilocytic astrocytoma grade I [PA] vs diffuse astrocytoma grade II vs nonpilocytic grade I/nondiffuse grade II vs no/before histology).
For PFS we also analyzed the time from diagnosis to start of therapy and dissemination even before start of therapy. Because resections were not always performed directly after diagnosis, the extents of resection and histology were included as time-dependent variables for OS and EFS. Nearly all resections took place before the start of adjuvant therapy. For PFS, we analyzed the extent of resection and histology as non-time-dependent variables. Thus patients with resections after start of therapy (n = 13) were considered as being without resections at the beginning of therapy. Interactions of main effects with time-dependent variables were tested in a second block. For OS, the model with interactions did not converge (as there were only 59 events), and no interaction term showed further influence on EFS.
Given for the final models are estimated hazard ratios of the selected explanatory variables with their respective 95% confidence intervals and likelihood ratio test P values. All children meeting the eligibility criteria of the HIT-LGG-1996 study were planned to be included in this trial to assess the feasibility of a comprehensive treatment strategy. Thus, all analyses were regarded as explorative, and P values are given descriptively to detect and study meaningful effects. Therefore, no significance level was fixed. Analyses were performed by the Institute for Medical Biostatistics, Epidemiology, and Informatics.
Response Assessment
Neuroradiologic assessment of tumor response by MRI was scheduled at weeks 12, 24, 36, and 48 and after week 53 following the start of chemotherapy. During primary observation as well as after the completion of radiotherapy and chemotherapy, regular surveillance imaging was recommended with gradually increasing intervals. Radiologic assessment of tumor response by MRI followed recommended criteria.29 Considered positive were complete response (CR), partial response (PR), objective response (OR), and stable disease (SD).
Clinical and ophthalmologic assessments were recommended on a comparable time frame. Their results were not recorded systematically.
Results
Patients
One hundred five institutions took part in the study: 91 in Germany, 7 in Austria, 6 in Switzerland, and 1 in Belgium, registering 1182 patients between October 1, 1996, and March 31, 2004. Fifty-five patients had been diagnosed prior to the start of the trial (October 3, 1988, to September 9, 1996) but had not been treated and were included in the trial for observation (26) or for treatment of progression during the trial period (29). Ninety-nine patients were excluded for the following reasons: prior nonsurgical treatment (55), lack of consent (19), nontrial diagnoses (14), age >17 years (8), and LGG as second tumor (3). An additional 30 patients were excluded for missing data. Following tumor progression after the end of the recruitment phase, 22 patients from the observation group started nonsurgical therapy according to the SIOP (International Society of Pediatric Oncology)-LGG 2004 protocol and were excluded from analysis.
Thus, this report included 1031 patients (902 from Germany, 92 from Austria, 30 from Switzerland, and 7 from Belgium). The last follow-up was obtained in June 2011. Countrywide registration had its limitations in documenting details of initial symptoms and their evolutions during long-term follow-up.
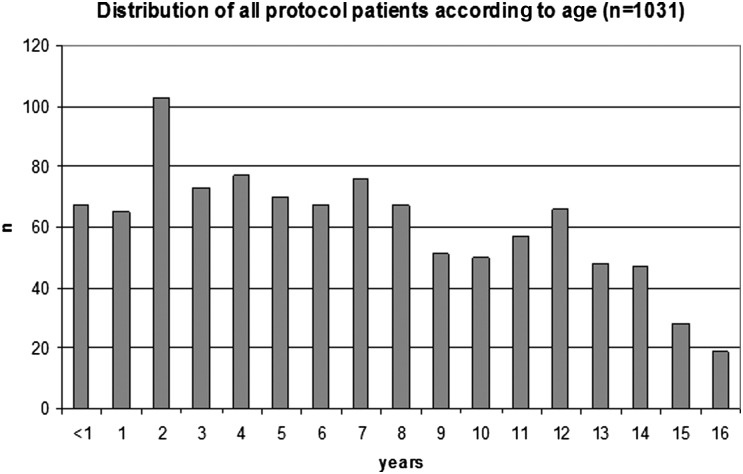
Patient data are detailed in Table 1. Median age at diagnosis was 6.9 years (Fig. 1). The sex ratio showed a slight male preponderance (1.12). Of the 1031 children, 108 (10.5%) met the diagnostic criteria of the US National Institutes Health for NF-1, and 17 (1.6%) had signs of tuberous sclerosis complex (TSC). Patients with NF-1 had comparable age at diagnosis (median, 5.1 y; range, 1.0–15.4 y) and a sex ratio of 56 females: 52 males. By clinical definition, 28 children had DS,30–32 with hypothalamic-chiasmatic glioma (26/28) and a high frequency of primary dissemination (6/28). Their median age at diagnosis was 6.5 months (range, 2.4–27.1 mo; 23/28 <1 y).
Table 1.
Patient data detailed for the complete trial cohort and observation as well as chemotherapy and radiotherapy treatment groups
| All Patients | Observation | Treatment Category (363/1031 = 35.2%) |
||
|---|---|---|---|---|
| Chemotherapy | Radiotherapy | |||
| Number of patients | 1031 | 668 | 216 | 147 |
| Sex | ||||
| Male | 545 | 370 | 100 | 75 |
| Female | 486 | 298 | 116 | 72 |
| Phakomatoses | ||||
| No | 903 | 606 | 161 | 136 |
| NF-1 | 108 | 43 | 55 | 10 |
| TSC | 17 | 16 | – | 1 |
| Not known | 3 | 3 | – | – |
| Age at diagnosis (y) | ||||
| Median | 6.9 | 7.8 | 3.2 | 8.7 |
| Range | 0.04–17.02a | 0.04–17.02a | 0.2–16.3 | 0.6–16.9 |
| Localization | ||||
| Cerebral hemisphere | 187 | 157 | 10 | 20 |
| Supratentorial midline | 417 | 156 | 171 | 90 |
| Optic nerve | 28 | 14 | 12 | 2 |
| Chiasm ± dorsal extension | 203 | 44 | 129 | 30 |
| Diencephalon | 114 | 58 | 21 | 35 |
| Mesencephalon | 70 | 39 | 8 | 23 |
| Not determined | 2 | 1 | 1 | – |
| Posterior fossa | 377 | 320 | 22 | 35 |
| Cerebellum | 291 | 267 | 8 | 16 |
| Caudal brainstem | 86 | 53 | 14 | 19 |
| Spinal cord | 36 | 22 | 13 | 1 |
| Lateral ventricle | 13 | 12 | – | 1 |
| Not determined | 1b | 1b | – | – |
| Histology | ||||
| Astrocytic tumors | 801 | 520 | 154 | 127 |
| PA I°,c | 700 | 455 | 137 | 108 |
| SEGA I° | 15 | 14 | – | 1 |
| A II° | 59 | 33 | 12 | 14 |
| PXA II° | 13 | 12 | – | 1 |
| LGG NOS | 14 | 6 | 5 | 3 |
| O II° | 10 | 7 | 2 | 1 |
| OA II° | 5 | 4 | – | 1 |
| GG I°, DNT, other | 92 | 77 | 7 | 8 |
| Histology not clarified | 8 | 5 | 1 | 2 |
| No histology | 115 | 55 | 52 | 8 |
| Extent of first resection | ||||
| Complete resection | 359 | 343 | 8 | 8 |
| Subtotal resection | 195 | 141 | 30 | 24 |
| Partial resection | 158 | 75 | 53 | 30 |
| Biopsy | 204 | 54 | 73 | 77 |
| Open | 106 | 36 | 51 | 19 |
| Stereotactic | 98 | 18 | 22 | 58 |
| No resection | 115d | 55 | 52d | 8 |
Histology according to WHO classification48,49. Abbreviations: PA I°, pilocytic astrocytoma I°; SEGA I°, subependymal giant cell astrocytoma I°; A II°, astrocytoma II° (diffuse); LGG NOS, low-grade astrocytic glioma not otherwise specified; PXA, pleomorphic xanthoastrocytoma II°; O II°, oligodendroglioma II°; OA II°, oligoastrocytoma II°; GG I°, DNT, other, ganglioglioma I°, dysembryoplastic neuroepithelial tumor, and other mixed glioneuronal tumors.
a One patient was radiologically diagnosed prior to age 17 y but had confirmatory histology at age 17.02 y.
b One patient had no definable site of tumor origin.
c Retrospectively, 5 patients with pilomyxoid astrocytoma were identified among PAs.
d One patient had a stereotactic biopsy yielding no tumor tissue and therefore was counted as having no histology.
Fig. 1.
Age distribution (y) at diagnosis (n = 1031). Median patient age is 6.9 y; interquartile range, 3.2–11.1 y.
The majority of tumors (40.4%) were located in the supratentorial midline (SML). Number of VP gliomas (22.4%) exceeded that of lesions from other regions of the diencephalon and mesencephalon. Associated with TSC were 9/13 tumors (1.3%) of the lateral ventricles. One tumor had no definable localization.
Primary tumor dissemination was found in 22 children and secondary tumor dissemination was found in another 22, and 2 were not defined. Children with primary dissemination were young (median age 2.6 years) with an age range up to 15.6 years. The majority of primary tumors were PAs (17/22) located in the hypothalamic-chiasmatic and other diencephalic regions (16/22). Nodules or laminar layers were found in the spinal (21) or cerebral subarachnoid spaces (13) or both (9) (with 1 not known).33
Initial surgical intervention achieved complete resection in 34.8% of patients, 204 tumors underwent biopsy only (19.8%), and 115 children received a diagnosis of tumor on the basis of neuroradiologic findings (11.2%), with 65 being NF-1. Of the 115 diagnoses, 106 were located in the SML (79/115 in VPs, 18/79 in the optic nerve only), 7 in the posterior fossa, and 1 in the cerebral hemispheres, with 1 not definable. Radiologic review was conducted in 108 cases. Extent of resection interacted with tumor location (Table 2).
Table 2.
Distribution of patients, according to tumor localization with respect to extent of resection and to histology
| Localization |
||||||
|---|---|---|---|---|---|---|
| Cerebral Hemisphere | Supratentorial Midline | Cerebellum | Caudal Brainstem | Spinal Cord | Lateral Ventricle/other | |
| Extent of resection | ||||||
| Complete | 100 | 35 | 191 | 16 | 10 | 7 |
| 96/1/3 | 29/4/2 | 188/1/2 | 15/-/1 | 8/2/- | 7/-/- | |
| Subtotal | 41 | 51 | 66 | 30 | 3 | 4 |
| 34/3/4 | 25/20/6 | 55/2/9 | 21/4/5 | 2/1/- | 4/-/- | |
| Partial | 20 | 86 | 21 | 15 | 15 | 1 |
| 12/3/5 | 30/38/18 | 14/3/4 | 8/4/3 | 10/5/- | 1/-/- | |
| Biopsy | 25 | 139 | 11 | 20 | 8 | 1 |
| 14/3/8 | 25/58/56 | 8/2/1 | 5/5/10 | 2/5/1 | 1/-/1 | |
| Radiologic diagnosis | 1 | 106 | 2 | 5b | - | 1c |
| 1/-/- | 47/51/8 | 2/-/- | 4/1b/- | -/-/- | 1c/-/- | |
| Histology | ||||||
| Pilocytic astrocytoma I° | 62 | 262 | 270 | 72 | 31 | 3 |
| 47/5/10 | 89/105/68 | 251/5/14 | 45/12/15 | 20/10/1 | 3/-/- | |
| Giant cell astrocytoma I° | 2 | 3 | - | - | - | 10 |
| 2/-/- | 3/-/- | -/-/- | -/-/- | -/-/- | 9/-/1 | |
| Diffuse astrocytoma II° | 23 | 24 | 8 | 3 | 1 | - |
| 18/2/3 | 8/6/10 | 5/3/- | 2/-/1 | -/1/- | -/-/- | |
| LGG not otherwise specified | 4 | 5 | 1 | 2 | 2 | - |
| 3/-/1 | 1/2/2 | 1/-/- | 1/1/- | -/2/- | -/-/- | |
| Oligoastrocytoma II° | 4 | - | 1 | - | - | - |
| 3/-/1 | -/-/- | 1/-/- | -/-/- | -/-/- | -/-/- | |
| Oligodendroglioma II° | 7 | 3 | - | - | - | - |
| 7/-/- | -/2/1 | -/-/- | -/-/- | -/-/- | -/-/- | |
| Pleomorphic xanthoastrocytoma II° | 13 | - | - | - | - | - |
| 12/-/1 | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | |
| Glioneuronal tumors | 68 | 11 | 8 | 3 | 2 | - |
| 61/3/4 | 6/4/1 | 6/-/1 | 1/-/2 | 2/-/- | -/-/- | |
| Radiologic diagnosis | 1 | 106 | 2 | 5 | - | 1c |
| 1/-/- | 47/51/8 | 2/-/- | 4/1/- | -/-/- | 1c/-/- | |
| Histology not clarified | 3 | 3 | 1 | 1 | - | - |
| 3/-/- | 2/1/- | -/-/1 | -/-/1 | -/-/- | -/-/- | |
a First line: all cases; second line: observation/chemotherapy/radiotherapy group.
b One patient had a stereotactic biopsy yielding no tumor tissue and therefore is counted as having no histology.
c One patient had no definable site of tumor origin.
The majority of histologic diagnoses were PAs (67.9%). Among 101 grade II tumors (9.8%), diffuse astrocytoma prevailed (59). Mixed glioneuronal histology was identified in 92 tumors (8.9%). The distribution of histologies within the CNS regions is detailed in Table 2.
For 539 (58.8%) of 916 cases, histologies were centrally reviewed, with a growing compliance over the years. In 68 (12.6%) of these 539 cases, variable degrees of discrepancy within the category of LGG were noted, being lowest for the group of hypothalamic-chiasmatic tumors (6/95, 6.3%). Pilomyxoid astrocytoma grade II was retrospectively identified in 5 specimens of PA.
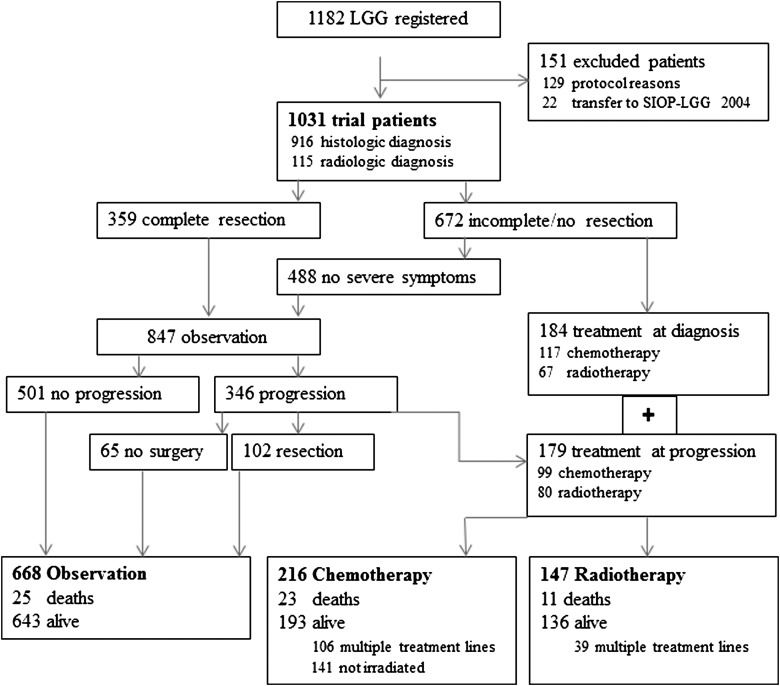
Distribution among the Strategy Groups
Following first surgical intervention in 916 children and radiologic diagnosis in 115 children, 847 patients were observed and 184 proceeded to nonsurgical treatment at diagnosis (1 without exact date). Indications for immediate therapy were severe neurologic symptoms (67), visual impairment (74), and risk for tumor progression at a critical site (10); several patients (5) had tumors suitable for brachytherapy; for patients (16) without symptoms or progression, therapy was initiated at the discretion of the treatment center; a small number of cases (12) were not detailed.
While on observation, 346 children had relapse or progression. Nonsurgical therapy was indicated in 179 children; and 167 remained in the observation arm, of whom 12 died early from progression. Following surgical intervention, 94 of the 167 patients survived with (40) or without (54) residual tumor, and 5 later died (3 not known). The remaining patients were observed without intervention, and 45 survived with (39) or without (6) residual tumor, while 8 later died.
Among 359 patients with initially completely resected tumors, 54 had relapse. Twelve patients received nonsurgical treatment (3.3%). The others remained observed with (26) or without (16) secondary surgical intervention, all surviving with SD. The completeness of initial resection and the extent of relapse were not centrally reviewed.
At the close of the study, 668 children were under observation and 363 had started adjuvant treatment, as either chemotherapy or radiotherapy (Fig. 2). Compared with the radiotherapy group, patients in the chemotherapy group were younger and more often had NF-1, any resection but fewer biopsies, and more frequent SML lesions but fewer lesions of the hemispheres or of the posterior fossa. Thus, further results are presented separately for the nonsurgical treatment groups.
Fig. 2.
Study strategy and distribution of patients throughout the course of the trial.
Radiotherapy
Of the 147 children (including 10 with NF-1 and 1 with TSC) who received radiotherapy for their primary tumor, 96 were treated with external beam fractionated radiotherapy (including gamma-knife in 1), and 51 underwent brachytherapy with iodine-125 seeds (including 3 with NF-1 and 1 with TSC). Treatment started within 3 months of diagnosis in 67 patients and following observation for 3–141 months in 80 patients (median, 9.4 mo). Median patient age was 8.7 years (range, 0.6–16.9 y) at diagnosis and 9.8 years (0.7–18.8) at start of therapy. Six children were <1 year of age, 4 had disseminated disease, and 2 had DS.
Best tumor responses to irradiation were CR in 13.7%, PR in 34.4%, and OR/SD in 42.7%, while 9.2% of tumors progressed (131/147 evaluable). Best radiologic responses were documented after a median interval of 5.7 months (range, 1–37.3 mo).
During follow-up there were 50 events. Progression within the first year was seen in 3/4 children with primary disseminated LGG. Tumor progressions occurred in 16/51 after iodine-seed brachytherapy and in 32/96 subsequent to external beam radiotherapy (9/48 died). One child died of tumor-related complications and 1 from a lethal accident. Thus 11/50 children died in the radiotherapy group. Progression or death occurred after a median interval of 1.1 years (range, 0.1–7.3 y, n = 49) after the start of treatment.
Chemotherapy
Vincristine + carboplatin chemotherapy was administered to 216 children (55 with NF-1). Treatment started within 3 months of diagnosis in 115 and following observation for 3–158 months (median, 14.0 mo) in 99, with 2 not documented. Median age was 3.2 years (range, 0.2–16.3 y) at diagnosis and 4.3 years (range, 0.3–19.8) at start of therapy for those initially observed.
Best tumor responses were CR in 3.8%, PR in 31.6%, and OR/SD in 56.5%, while 8.1% had tumor progression (209/216 evaluable). Best radiologic responses were documented after a median interval of 3.4 months (range, 0.8–33.4 mo, n = 209).
During follow-up there were 130 events. Progression or death occurred after a median interval of 1.9 years (range, 0.1–11.8 y) from the start of treatment, with 33 events developing during the 1-year treatment time.
There was 1 toxic death during chemotherapy and 1 infectious death during salvage chemotherapy. Death from tumor progression occurred to 18 patients, and 2 died from tumor-related complications (one after prior progression). For 1 child who had multiple progressions, the exact cause of death is not known. Thus 23/130 children died in the chemotherapy arm.
Treatment toxicity
Treatment-associated toxicity was not recorded for the radiotherapy group but was documented for the chemotherapy group in the major categories.
Although half of the 216 chemotherapy patients experienced grade III and IV hematotoxicity, only a few had relevant ototoxicity or nephrotoxicity. Allergy to carboplatin was reported in 78 (53%) of the 147 evaluable patients in the chemotherapy group and was managed individually, but 30 (38%) of these 78 children continued treatment without any change. In 39 patients, treatment was switched to cyclophosphamide, cisplatin, or other agents at various time points, and 6 terminated treatment early (no information for 3 patients).
Survival analysis
Overall survival
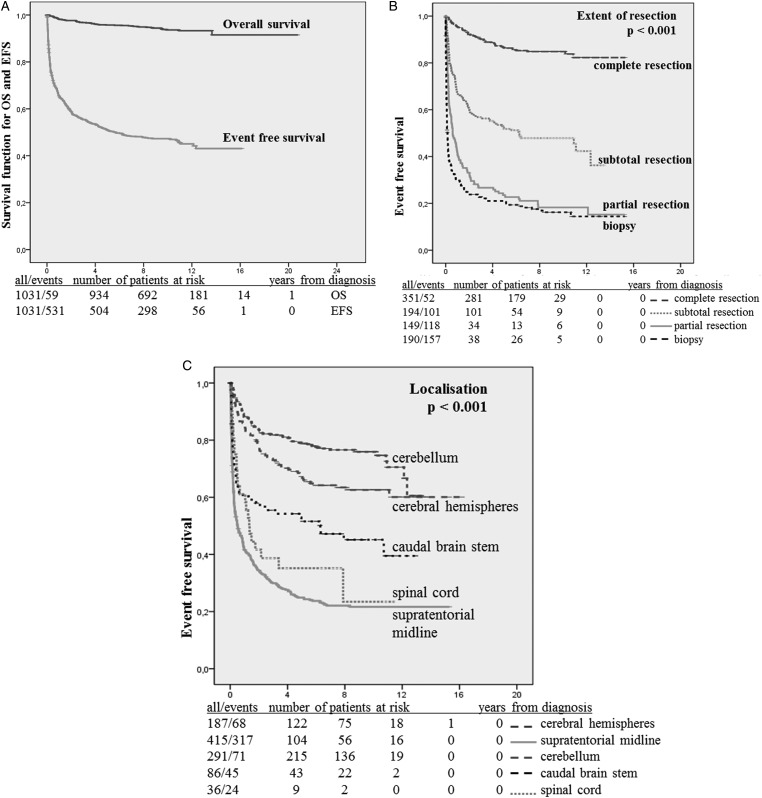
At a median observation time of 9.3 years (range, 0–20.8 y), 5- and 10-year OS values were 0.96 and 0.94 (59 events) (Fig. 3A). Death occurred to 43 children from tumor progression, to 7 from tumor-related complications, and to 2 from unrelated accidents. There was 1 death due to toxicity (in the chemotherapy group), and 6 patients died at home without documented cause.
Fig. 3.
(A) Overall survival (OS) and event-free survival (EFS) for all patients (n = 1031). (B) EFS in relation to the extent of first resection. (c) EFS in relation to primary tumor site.
OS was lower for children <1 year of age compared with the other age groups, in cases of dissemination at diagnosis, of DS, and of partial resection or biopsy only. Patients with tumors in the spinal cord or SML and with nonpilocytic histology had a slightly inferior prognosis (Table 3).
Table 3.
Ten-year overall and event-free survival (univariate analysis)
| Overall Survival |
Event-free Survival |
|||||
|---|---|---|---|---|---|---|
| No. | 10-y OS | Log-rank P value | No. | 10-y EFS | Log-rank P value | |
| All | 1031 | 0.94 | - | 1031 | 0.49 | |
| Chemotherapyb | 215 | 0.88 | ||||
| Radiotherapy | 147 | 0.91 | ||||
| Sex | 0.296 | 0.23 | ||||
| Female | 486 | 0.95 | 486 | 0.45 | ||
| Male | 545 | 0.93 | 545 | 0.49 | ||
| NF-1a | 0.587 | <0.001 | ||||
| No | 903 | 0.94 | 903 | 0.5 | ||
| Yes | 108 | 0.95 | 108 | 0.24 | ||
| Age, y | <0.001 | <0.001 | ||||
| <1 | 67 | 0.73 | 67 | 0.19 | ||
| 1–4 | 317 | 0.95 | 317 | 0.42 | ||
| 5–10 | 381 | 0.98 | 381 | 0.5 | ||
| ≥11 | 266 | 0.92 | 266 | 0.57 | ||
| Histologya | 0.04 | <0.001 | ||||
| Pilocytic astrocytoma I° | 697 | 0.94 | 671 | 0.5 | ||
| Diffuse astrocytoma II° | 59 | 0.87 | 59 | 0.4 | ||
| Nonpilocytic °I/nondiffuse °II | 149 | 0.92 | 146 | 0.61 | ||
| Disseminated LGGa | <0.001 | <0.001 | ||||
| Present | 22 | 0.67 | 22 | 0 | ||
| Absent | 1007 | 0.94 | 1007 | 0.49 | ||
| DS | <0.001 | <0.001 | ||||
| Present | 28 | 0.47 | 28 | 0 | ||
| Absent | 1003 | 0.95 | 1003 | 0.49 | ||
| Localizationa | 0.002 | <0.001 | ||||
| Cerebral hemispheres | 187 | 0.95 | 187 | 0.63 | ||
| Supratentorial midline | 415 | 0.9 | 415 | 0.22 | ||
| Posterior fossa | 377 | 0.98 | 377 | 0.69 | ||
| Spinal cord | 36 | 0.91 | 36 | 0.23 | ||
| Lateral ventricle | 13 | 0.9 | 13 | 0.62 | ||
| Extent of resectiona | <0.0001 | <0.001 | ||||
| Complete | 351 | 0.99 | 354 | 0.85 | ||
| Subtotal | 194 | 0.94 | 197 | 0.48 | ||
| Partial | 149 | 0.87 | 152 | 0.18 | ||
| Biopsy | 190 | 0.88 | 192 | 0.16 | ||
| No resection | 115 | c | 115 | c | ||
OS and EFS calculated from date of diagnosis (to evaluate the variable extent of resection and histology: calculated from date of resection; to evaluate therapy groups: calculated from date of start of therapy.)
a Missing values to add up to 1031: NF-1: 20 patients had other phakomatoses, or NF-status was not clarified; all other categories: missing information.
b Chemotherapy group: for 1 patient the exact date of start of treatment is not known.
c Estimate for OS/EFS from date of resection not evaluable due to no resection.
Multivariate analysis indicated that age >11 years and DS, which segregates with infants, were unfavorable factors, as was incomplete resection (Table 4).
Table 4.
Results of the multivariate analysis
| Comparison | Hazard Ratio (95% CI) | P value* | |
|---|---|---|---|
| Overall survival (n = 987; 57 deaths) | |||
| Diencephalic syndrome | Present vs absent | 4.58 (1.84; 11.40) | 0.001 |
| Age at diagnosis | <1 vs 1–4 y | 2.10 (0.85; 5.18) | 0.002 |
| 5–10 vs 1–4 y | 0.53 (0.22; 1.32) | ||
| ≥11 vs 1–4 y | 2.32 (1.84; 11.40) | ||
| Extent of resection | Complete vs no/before resection | 0.18 (0.04; 0.94) | <0.001 |
| Subtotal vs no/before resection | 1.49 (0.54; 4.42) | ||
| Partial vs no/before resection | 2.88 (1.06; 7.84) | ||
| Biopsy vs no/before resection | 2.42 (0.90; 6.49) | ||
| Event-free survival (n = 996; 514 events) | |||
| Diencephalic syndrome | Present vs absent | 3.15 (2.08; 4.75) | <0.001 |
| Extent of resection | Complete vs no/before resection | 0.30 (0.21; 0.44) | <0.001 |
| Subtotal vs no/before resection | 1.23 (0.92; 1.65) | ||
| Partial vs no/before resection | 2.37 (1.80; 3.11) | ||
| Biopsy vs no/before resection | 3.48 (2.68; 4.53) | ||
| Localization | Hypothalamic-chiasmatic vs other | 2.91 (2.35; 3.69) | <0.001 |
| Other di/mesencephalic vs other | 1.66 (1.27; 2.16) | ||
| Progression-free survival postradiotherapy (n = 138; 48 events) | |||
| Dissemination at diagnosis | Present vs absent | 19.57 | 0.006 |
| (4.06; 94.50) | |||
| Histology at start of therapy | No histology vs PA I° | 1.18 (0.27; 5.14) | <0.001 |
| Diffuse A II° vs PA I° | 0.87 (0.30; 2.54) | ||
| Nonpilocytic I°/nondiffuse A II° vs PA I° | 6.80 (3.20; 14.42) | ||
| Age at diagnosis | <1 vs 1–4 y | 4.91 (1.60; 15.10) | 0.007 |
| 5–10 vs 1–4 y | 0.76 (0.31; 1.86) | ||
| ≥11 vs 1–4 y | 1.53 (0.61; 3.84) | ||
| Progression-free survival postchemotherapy (n = 210; 126 events) | |||
| Diencephalic syndrome | Present vs absent | 1.96 (1.09; 3.54) | 0.032 |
| Dissemination prior to therapy | Present vs absent | 2.03 (1.22; 3.37) | 0.010 |
| Sex | Boys vs girls | 0.62 (0.43; 0.89) | 0.009 |
| Age at diagnosis | <1 vs 1–4 y | 1.12 (0.68; 1.87) | 0.004 |
| 5–10 vs 1–4 y | 0.52 (0.31; 0.88) | ||
| ≥11 vs 1–4 y | 1.85 (1.01; 3.38) | ||
| NF1 | Present vs absent | 0.58 (0.36; 0.89) | 0.021 |
Boldface indicates hazard ratios with confidence intervals not including 1.0.
*P value of the likelihood ratio test.
Event-free survival
Of the 1031 children, 531 had events resulting in 5- and 10-year EFS values of 0.51 and 0.47, respectively (Fig. 3A). Events were observed as late as 12.3 years postdiagnosis.
EFS was determined by the extent of resection and primary tumor location. It was best following complete resection and worst after biopsy (P < .001) (Fig. 3B). Patients with SML lesions or lesions of the spinal cord had lower EFS than patients with tumors in the cerebral hemispheres, the posterior fossa, or other sites (P < .001) (Fig. 3C). Tumor site interacted with resection even for extent of complete resection, resulting in 10-year EFS values of 0.92 at the cerebellum, 0.80 at hemispheres, 0.69 at SML, and 0.58 at spinal cord (Table 5).
Table 5.
Ten-year event-free survival, univariate analysis
| Localization | Extent of Resection |
Log-rank | ||
|---|---|---|---|---|
| Complete Resection | Subtotal/Partial Resection | Biopsy | ||
| Cerebral hemisphere (n = 183) (1 no biopsy, 3 missing) | 0.80 (n = 99) | 0.52 (n = 61) | 0.28 (n = 23) | <0.001 |
| Supratentorial midline (n = 284) (106 no biopsy, 27 missing) | 0.69 (n = 29) | 0.18 (n = 129) | 0.11 (n = 126) | <0.001 |
| Cerebellum (n = 286) (2 no biopsy, 3 missing) | 0.92 (n = 188) | 0.48 (n = 87) | 0.55 (n = 11) | <0.001 |
| Caudal brainstem (n = 80) (5 no biopsy, 1 missing) | 0.87 (n = 16) | 0.41 (n = 44) | 0.16 (n = 20) | <0.001 |
| Spinal cord (n = 33) (3 missing) | 0.58 (n = 10) | 0.00 (n = 16) | 0.14 (n = 7) | 0.068 |
For extent of resection as related to localization, calculated from date of resection.
NF-1 status conferred a high risk for progression postdiagnosis (10-year EFS of 0.24 vs 0.50 for non–NF-1 patients). Patients with dissemination, DS, or non-PA histology or children aged <1 year at diagnosis had significantly impaired EFS (Table 3).
Multivariate analysis confirmed that the risk for event was determined by site (unfavorable were all SML locations, P < .001), extent of first surgical resection (favorable was complete resection; unfavorable was partial resection or biopsy, P < .001), and presence of DS (P < .001), while no influence was found for age, sex, NF-1 status, histology, or dissemination (Table 4).
Progression-free survival: efficacy of adjuvant treatment
Radiotherapy
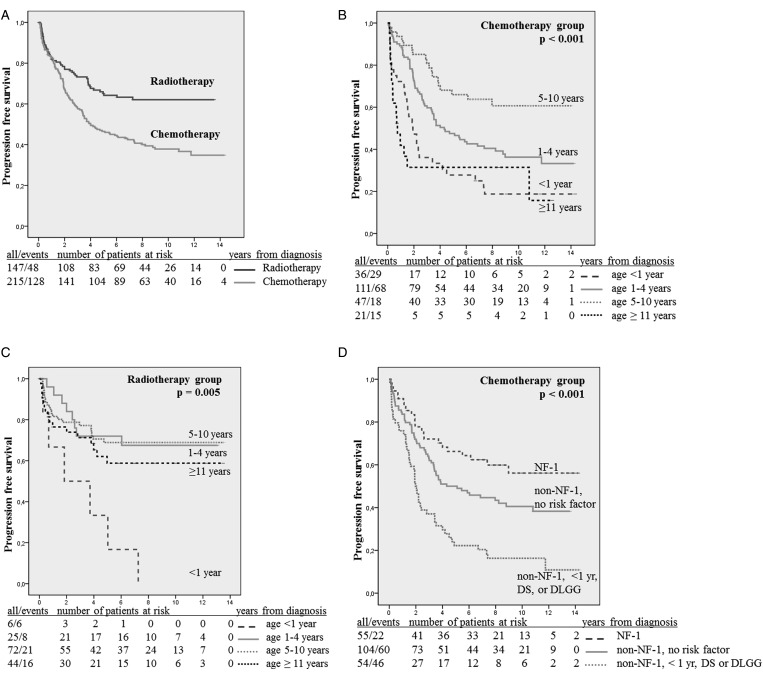
After a median observation time from diagnosis of 9.5 years (range, 0.1–19.9 y) and time from end of therapy of 8.3 years (range, 0.0–13.5), 5- and 10-year PFS values following the start of treatment were 0.65 and 0.62 (n = 147) (Fig. 4A).
Fig. 4.
(A) Progression-free survival (PFS) for the radiotherapy and chemotherapy groups. (B) PFS within the chemotherapy group according to age subgroup (<1, 1–4, 5–10, and ≥11 y). (C) PFS within the radiotherapy group according to age subgroup (<1, 1–4, 5–10, and ≥11 y). (D) PFS within the chemotherapy group in relation to NF-1 status and risk factors: NF-1 patients (n = 55) vs non–NF-1 patients aged >1 y at diagnosis, no dissemination, no DS (n = 104) vs non–NF-1 patients <1 y, or with dissemination or with DS (n = 54), 10-y PFS: 0.56 vs 0.41 vs 0.16. Abbreviations: NF-1, neurofibromatosis type I; DS, diencephalic syndrome; DLGG, disseminated low-grade glioma.
Univariate analysis showed that age (Fig. 4C), DS, dissemination, and histology modulated PFS. All children aged <1 year and those with DS had progression, as did 3/4 patients with metastases at diagnosis (P = .027). PFS was higher for patients with PAs and diffuse astrocytomas grade II but lower for patients with nonpilocytic grade I/nondiffuse grade II lesions (P < .001) (Table 6).
Table 6.
Ten-year progression-free survival: chemotherapy and radiotherapy group
| Risk Factor | Chemotherapy* |
Radiotherapy |
||||
|---|---|---|---|---|---|---|
| No. | 10-y PFS | Log-rank P value | No. | 10-y PFS | Log-rank P value | |
| Sex* | 0.27 | 0.209 | ||||
| Male | 99 | 0.47 | 75 | 0.54 | ||
| Female | 116 | 0.3 | 72 | 0.7 | ||
| NF 1* | 0.001 | 0.764 | ||||
| No | 160 | 0.32 | 136 | 0.62 | ||
| Yes | 55 | 0.56 | 10 | 0.66 | ||
| Age* | <0.001 | 0.005 | ||||
| <1 y | 36 | 0.19 | 6 | 0 | ||
| 1–4 y | 111 | 0.36 | 25 | 0.68 | ||
| 5–10 y | 47 | 0.61 | 72 | 0.69 | ||
| ≥11 y | 21 | 0.31 | 44 | 0.59 | ||
| Histology | 0.673 | <0.001 | ||||
| Pilocytic astrocytoma °I | 126 | 0.32 | 104 | 0.65 | ||
| Diffuse astrocytoma °II | 12 | 0.42 | 14 | 0.7 | ||
| Nonpilocytic °I/nondiffuse °II histology | 13 | 0.39 | 15 | 0.27 | ||
| Disseminated LGG (at diagnosis) | <0.001 | 0.027 | ||||
| Present | 22 | 0.1 | 4 | 0.25 | ||
| Absent | 190 | 0.41 | 141 | 0.64 | ||
| Diencephalic syndrome | <0.001 | 0.054 | ||||
| Present | 21 | 0.1 | 2 | 0 | ||
| Absent | 194 | 0.41 | 145 | 0.63 | ||
| Localization | 0.322 | 0.866 | ||||
| Hypothalamic-chiasmatic | 128 | 0.38 | 30 | 0.7 | ||
| Other diencephalon | 26 | 0.42 | 53 | 0.61 | ||
| Cerebral hemispheres/cerebellum/caudal brainstem/spinal cord | 42 | 0.33 | 50 | 0.63 | ||
| Extent of resection at start of therapy* | 0.201 | 0.809 | ||||
| Complete | 7 | 0.38 | 6 | 0.44 | ||
| Subtotal | 29 | 0.35 | 23 | 0.69 | ||
| Partial | 47 | 0.32 | 28 | 0.65 | ||
| Biopsy | 69 | 0.33 | 75 | 0.66 | ||
| No histology or preresponse | 62 | 0.5 | 10 | 0.75 | ||
| Response week 24 | 0.2 | |||||
| PR/CR | 18 | 0.6 | Not done | |||
| OR/SD | 92 | 0.45 | ||||
Calculated from the date of start of therapy (in case of response at week 24: calculated from date of response) (univariate analysis).
*Missing values to add up to 216 in the chemotherapy and 147 in the radiotherapy group: NF-1: 1 patient had TSC; all other categories: missing information; in the chemotherapy group: 1 patient without date for start of therapy.
Multivariate analysis included 138 patients (48 events). The risk for tumor progression following radiotherapy was increased in cases of nonpilocytic grade I/nondiffuse grade II lesions, of patient age <1 year, and of dissemination at diagnosis. Sex, NF-1 status, location, extent of resection, and presence of DS had no independent influence (Table 4). The unfavorable results for children with disseminated LGG required individual case analysis.33
Chemotherapy
After a median observation time from diagnosis of 10.4 years (range, 0.2–18.6 y) and of 8.6 years (range, 0.1–13.4) from end of therapy, 5- and 10-year PFS values following the start of treatment were 0.47 and 0.44 (Fig. 4A).
For 128 patients with VP gliomas, 10-year PFS was 0.35. PFS was better for patients with NF-1 than for those without (0.46 vs 0.30) and for patients aged >1 year than for younger patients (0.41 vs 0.18).
Univariate analysis showed numerous influential factors for risk for progression or death following chemotherapy (Table 6). Ten-year PFS values were 0.19 for children aged <1 year and 0.36 for children aged 1–4 years. Best PFS of 0.61 was found in the group of 5- to 10-year-old patients, dropping to 0.31 for patients ≥11 years (P < .001) (Fig. 4B). Lack of tumor dissemination at start of therapy was associated with a higher PFS than was proof of it (P < .001). Similarly, cases without DS had a better PFS than cases with DS (P < .001). NF-1 patients had a better prognosis following treatment than non–NF-1 patients (P = .001); they lacked dissemination or DS and were age >1 year. PFS in the NF-1 cohort remained better, even when we excluded patients with these criteria in the non–NF-1 group (P < .0001, Fig. 4D).
Despite the protocol recommendation, only 110 children had an MRI study at 24 weeks following the start of chemotherapy. The extent of response at week 24 (CR/PR vs OR/SD) was not prognostic.
Multivariate analysis included 210 patients (126 events). Unfavorable independent prognostic variables for risk for tumor progression or death postchemotherapy were age >11 years, presence of tumor dissemination, and DS. Favorable factors were NF-1 status, age 5–10 years, and male sex. Location, histology, and extent of resection had no influence on PFS (Table 4).
Salvage Treatment
Among the 48 children with progression postradiotherapy (including 3/10 cases of NF-1), 26 went on to receive vincristine + carboplatin (22) or other (4) chemotherapy, 4 had second radiotherapy as brachytherapy (3) or local irradiation (1), and 6 remained under observation after surgical intervention (3 CR, 3 SD). A biopsy disclosed that 1 patient had glioblastoma multiforme (initial diagnosis had been pleomorphic xanthoastrocytoma with signs of anaplasia). Ten children had no intervention, 3 died, and the others attained SD (1 NF-1). There was no information for 1 patient.
For 8 patients, third-line chemotherapy was documented, and 3 received repeat radiotherapy as fourth-line treatment.
Among the 128 children with progression postchemotherapy (including 22/55 NF-1), 4 continued with vincristine + carboplatin, 52 received alternative chemotherapy regimens, and 45 (35.1%) went on to receive salvage radiotherapy with conventional external beam radiation; 10 children underwent surgery, with 7 attaining CR/PR and 3 SD. One of these progressed again and died; 14 children had no further treatment. While 9 tumors stopped growing and stabilized (2 NF-1), 5 children died from relentless progression (no NF-1). There was no information for 3 children.
Third-line treatment for 37 patients included irradiation in 11 and chemotherapy in 26, while fourth-line therapy was documented as drug treatment in 8 and irradiation in 7 (with 2 receiving second radiotherapy), and 2 cases were without details.
Following first or further progressions, 61 (28.2%) of 216 patients eventually received radiotherapy. For them, salvage radiotherapy had been delayed for 0.3–8.7 years (median, 2.7 y; n = 51). At the start of radiation, they had attained a median age of 7.2 years (range, 1.2–16.7 y, n = 45).
Discussion
Our cohort of pediatric LGG patients was unique in that at the time, no representative cohort of comparable size including LGGs of all locations and histologies was prospectively followed in a multicenter and multidisciplinary treatment strategy. Only the British series is now comparable.34 Inclusion of all centers reduces selection bias and documents the acceptability of our approach.
Epidemiology and comparability of the study cohort
Our observations of a slight male preponderance and a median age of 6.9 years with a peak at ages 2–5 are in line with previous studies.8,35–37
NF-1 children represented 10.5% of our cohort and these patients were reported elsewhere.38 The percentage of such patients is higher in some reports,34,39 whereas Fisher et al35 found only 5% in a retrospective review. NF-1 children have constituted 19.2%–31.7%40–43 of recent treatment series, which compares well with 25.5% of NF-1 patients in the chemotherapy arm of our study. TSC patients, who develop subependymal giant cell astrocytomas,44 represented 1.6% of our cohort. For both phakomatoses, we assume a referral bias, as only patients with tumors needing nonsurgical therapy may have been registered.
Cerebellar location, generally considered to be the most frequent site,5,8,18,34 ranked second to the SML region in our series. SML tumors accounted for 40.4% of cases, a proportion similar to that in the British cohort (39.1%),34 whereas 18%–25% were found in other series.6,37 This comes close to our 22.4% for VP glioma. However, in large infiltrative SML tumors, radiographic determination of the site of origin is often arbitrary.45 The relative incidence of tumors of other locations corroborates previous reports.46–49
We included LGGs of WHO grade I or II of astrocytic or oligodendrocytic origin20,21 and mixed glioneuronal tumors, if their glial component appeared relevant for diagnosis. As in most series,8,34,35,42 PAs constituted the majority of histologies. Pilomyxoid astrocytoma was described in 199950 but not included in the WHO classification prior to 2007.21,51 We retrospectively identified 5 patients originally diagnosed with PA variants in the SML and with median age at diagnosis of 0.46 year. Tumor progression caused death to 3 patients, indicating an aggressive subtype.
Distribution of histologies varies within the CNS (Table 2). In our cohort, glioneuronal tumors occurred at the same frequency as PAs in the cerebral hemispheres, and grade II gliomas made up almost 30%, whereas Pollack et al5 reported no glioneuronal tumors, and occurrence of grade II gliomas twice as frequently as PAs. Reports on the incidence of other histologies are comparable to our figures.36 Diffuse astrocytoma is the predominant entity within the group of infiltrating gliomas of WHO grade II, and its biologic behavior differs from that of the adult counterpart.52
Central histopathologic review at the German Brain Tumor Reference Center for Neuropathology was highly recommended, especially in difficult cases, and was performed in almost 60% of cases in our study. Shifts of diagnoses were frequent (12.6%). Concordance rates were even lower in other series37 or were not detailed.34
High-grade glioma was excluded, although 8 patients with an initial LGG developed high-grade glioma without (5) and with (3) prior nonsurgical treatment. Except for 1 initial astrocytoma not otherwise specified, all histologies were centrally reviewed. Malignant transformation is highlighted postradiotherapy,53 has been observed even without prior treatment,54–56 and is not restricted to the older age group57 or initial astrocytoma grade II.55 The frequency of <1% in our cohort is lower than incidence rates in smaller series.56 Malignant evolution may be linked to molecular genetic subtypes.52,58
In our study, no biopsy was performed in 11.2% of cases. For VP gliomas, MRI criteria combined with clinical information, such as NF-1 status, allowed diagnosis of the presence of an LGG and justified dispensing with a biopsy.59 Up to 40% unbiopsied LGGs were included in previous series of VP gliomas,34,39–42,60 with lower percentages reported in other locations, although outside the VPs, LGG histology should be confirmed.61
Initial Surgical Management and Events Postdiagnosis
Primary safe tumor resection is the treatment of choice for LGG.8,49 The distribution of tumor sites in our study contributed to a rather low portion of complete resections, confirmed by postoperative imaging (Table 2).62 Complete resection rates ranged 30%–95% for hemispheric tumors5,8,9,15 and up to 80%–90% for cerebellar locations.1,9,63 Complete resection rates in other locations have been much lower, justification for which is debated in view of long-term morbidity.6,37,49,64–66 Observation was recommended following complete tumor resection, as well as in cases of incomplete or no surgery, if presented without significant clinical symptoms or progression.8,34,37,39,67
EFS declined to 0.47 at 10 years for our patients, a value comparable to those seen in retrospective series.37,39 Our definition of events postdiagnosis prior to any treatment included the necessity to start nonsurgical treatment for severe or progressive symptoms, as first introduced by Janss et al60 in 1995. This makes it difficult to equate EFS with the natural history of LGG as postulated by Stokland et al.34 DS ranks among the most severe clinical conditions at diagnosis; chemotherapy is recommended as immediate treatment30–32,68 and was given to 21/28 patients from our series.
Predominantly, EFS is a function of tumor site and extent of resection, remaining high after complete resection in our series, especially at the cerebellar (0.92) and cerebral hemispheric (0.80) sites. While 10-year EFS was 0.48 following subtotal resection, EFS values following partial resection and biopsy were similar, at 0.18 and 0.16, respectively. This finding is in line with results from other reports.5,8,37,69,70
We observed a higher progression rate for diffuse astrocytoma grade II. Still, histology was not predictive at multivariate analysis, as confirmed for LGGs within the cerebral hemispheres by Pollack et al.5 However, in the Children's Oncology Group (COG)/Pediatric Oncology Group study,70 as in the series by Fisher et al37 and Stokland et al,34 PFS was significantly unfavorable for diffuse astrocytoma. As the PFS analyses of Fisher et al and Stokland et al combined the period of observation prior to treatment and subsequent management,34,37 their findings are not comparable to the separate EFS and PFS definitions of our trial. Progression was frequent at the SML locations, with 91.6% of tumors incompletely resected or unbiopsied, specifically for NF-1–associated extensive VP gliomas, as opposed to small, anteriorly located tumors.39,67 The presence of DS acts as an additional, unfavorable factor.30–32,68
Indication for Nonsurgical Treatment and Age-dependent Stratification
Assignment of patients to adjuvant treatment has always considered clinical symptoms, since the biology of LGG remains unpredictable.19 However, attitudes toward initiation of treatment have changed over time.
During observation after complete resection, 15% of our patients relapsed, and 3.3% of this group ultimately underwent nonsurgical treatment. No comparable figures can be drawn from other large cohorts.34,37,39,71
Initially, unresectable residual tumor was the main indication for adjuvant therapy.5,70,72 Later, a tumor residue was not considered an indication per se, since overall survival was not impaired in patient series with delayed irradiation.35,73 Our and other protocols40,60,67,74 foresaw threatening neurologic and/or ophthalmologic symptoms as indications for nonsurgical treatment at diagnosis, particularly for small children and cases of SML lesions or NF-1. After initial observation, progression occurred in 40.9% of cases within 10 years and indicated nonsurgical therapy in 51.7%.
Ultimately, 35.2% of patients in our study received nonsurgical treatment. Within the British cohort, 25.8% started nonsurgical therapy,34 while a single center retrospectively reported a rate of 37.6%.39 In a French series,67 80% of 32 hypothalamic-chiasmatic tumors were eventually treated, a proportion comparable to the 62.6% for our SML tumors.
In accord with others, we aimed to define the efficacy of chemotherapy to delay or avoid radiotherapy for young children and reduce neurocognitive and endocrine impairment.11,65,72,75–79 We chose age >5 years as stratification for irradiation.11,60,80 Meanwhile, several reports published during the study period confirmed this approach.40–42,74 During recruitment, an increasing number of children aged >5 years received primary chemotherapy as a first-line option, and those patients eventually represented 31% of the chemotherapy cohort, corresponding to 40% of children aged >5 years in the COG protocol A9952.43
Even at progression, salvage chemotherapy was preferred for younger patients, as in other reports.19,71 Modern radiation equipment and especially brachytherapy, with its brain-sparing effect, 24,25,81–83 might permit safer treatment of younger patients with radiotherapy, depending upon tumor size and location.
Efficacy of Vincristine + Carboplatin Chemotherapy and Risk Factors for Progression
At the start of HIT-LGG-1996, various chemotherapeutic agents had been tested in small patient series for newly diagnosed or recurrent LGGs.11,40 In parallel with the British and Italian national groups,22 the GPOH adopted a regimen based on vincristine and single-dose carboplatin for a treatment duration of 1 year. The most bothersome unwanted effect of carboplatin-based regimens is allergy, which has occurred in 7%–78% of patients,23,84,85 whereas hematologic and nonhematologic toxicities appear tolerable.
As in other patient series, the response to nonsurgical treatment was assessed by radiologic criteria. One of the weaknesses of this series is lack of compulsory visual and/or neurologic documentation. Data concerning long-term ophthalmologic outcome postchemotherapy remain scant.42,86–88
The immediate response rate including disease stabilisation was high (91.9%) for our cohort, as also found by others.40–42,74 The “early” progression rate within the first year (33/216 patients) corroborated the findings of the contemporary series of Packer et al40 and Laithier et al.42 Supporting other reports, satisfactory PFS in the first years steadily declined without plateau (Fig. 4A), in contrast to an OS of 0.88 at 10 years (Table 3).42,43,60 Subsequent multiple-line salvage therapies, including surgery, chemotherapy, or radiotherapy for 86.7% of patients with progression in our study, were again effective in corroborating the findings of others.39,71,89,90 The intent to delay or avoid radiotherapy was successful. Radiotherapy was deferred for 0.3–8.7 years. Irradiated patients had a median age well above 5 years. Almost three-quarters of surviving chemotherapy-treated patients (141/193) have not yet received radiation treatment. No other cohort has been observed prospectively for so long. Radiotherapy was deferred for the majority of children in a retrospective Canadian report,71 but no timeline was provided. Radiation-free survival of 61% at 5 years' follow-up was reported for the French cohort of 84 patients by Laithier et al.42
Disease progression postchemotherapy has been analyzed by only univariate analysis in previous reports with smaller patient numbers or heterogeneous cohorts.40–42,74,91 Multivariate analysis of our data identified DS and tumor dissemination as unfavorable risk factors. While age group of 5–10 years, presence of NF-1, and male sex proved to be favorable, patients ≥11 years old had an increased risk for disease progression.
Young age, with variable cutoffs, has been identified as unfavorable in several recent reports.34,41–43,73 DS and leptomeningeal spread indicated a differing biology in smaller series.30,31,33,92 None of the 13 children with DS in the French Society of Pediatric Oncology study42 remained progression-free, and 5/9 children in the series by Poussaint et al30 developed recurrence. In contrast, in the series by Packer et al,40 only 2/7 children with DS had progression at short follow-up of 13–45 months. In our study, DS was the only parameter showing relevance in the Cox regression analysis for OS, EFS, and PFS after chemotherapy.
Leptomeningeal dissemination was documented in ∼5% of our patients, with dissemination present at initial diagnosis in 3%, confirming the findings of smaller cohorts.93–95 Details of this cohort are discussed separately.33
Very young children with hypothalamic-chiasmatic glioma seem to have the highest risk for tumor dissemination, with the risk even greater in cases of concurrent DS.33,92 In our chemotherapy group, the most unfavorable PFS was seen in cases of non-NF-1 with metastases at the start of treatment, or DS, or of age <1 year (n = 54) (Fig. 4D).
In contrast, children aged 5–10 years fared most favorably postchemotherapy, as recently confirmed for non–NF-1 patients in a randomized COG trial.43 PFS was even significantly superior, compared with that in children aged 1–4 years. Thus, arguments in favor of primary chemotherapy apply to both age groups.43
Age ≥11 years has not been addressed in prior chemotherapy reports. The cohort of Packer et al40 included patients ≤16 years and identified older age (>5 y) as an unfavorable prognostic parameter, though without giving the number of older children. Smaller series of 31 and 42 patients41,73,74 included adolescents ≤21 years and identified older age as favorable, though withholding details for children aged >10 years. Our cohort of children ≥11 years of age received primary chemotherapy, instead of radiotherapy, at the discretion of the treatment center. While tumor locations corresponded to the younger age groups (1–4 and 5–10 years) there were more infiltrating gliomas (5/20). However, numbers did not allow identification of histology as an independent unfavorable risk factor.
Similarly, no conclusions should be drawn from the more favorable prognosis for boys unless a sex-related prognosis is reproduced in other trials at long-term follow-up.
Multivariate analysis identified NF-1 status as a favorable factor in the chemotherapy arm, comprising 25.5% of patients. In concurrent reports, NF-1 disease was reported as unrelated to prognosis,40 as a positive predictor,41–43,60 and as unrelated to patient age.43 In our chemotherapy group, NF-1 status remained favorable in univariate analysis, even if children aged <1 year and those with dissemination or DS were excluded in the non–NF-1 subgroup. Neither of these risk factors segregated into the NF-1 cohort.38 NF-1 disease seems to confer a prolonged positive treatment response to chemotherapy despite a higher proportion of patients having progression postdiagnosis.
Efficacy of Radiotherapy and Risk Factors for Progression
Radiotherapy has been the mainstay of nonsurgical treatment of pediatric LGG for decades.19,82,83,96,97
Our radiotherapy cohort achieved a 5-year PFS of only 0.65, with a plateau of 0.62 at 10 years. PFS rates for brachytherapy and external beam radiotherapy were comparable.24,25 PFS remained at 0.72 for the subgroup of 132 children aged >1 year without dissemination or DS. Our results are inferior to those of other series reporting 5-year PFS rates of 80%, which included children with nonprogressive tumors.72,81,98,99
Multivariate analysis identified nonpilocytic grade I/nondiffuse grade II histology and dissemination at diagnosis as negative risk factors for PFS nonpilocytic grade I/nondiffuse grade II tumors had lower progression rates postdiagnosis, but if they did have progression, then their response to nonsurgical therapy was less favorable, and thus the outcome unfavorable. As this group harbors a variety of different histologies, the outcome has to be interpreted with caution.
No PFS difference was detected between cases of PA and of diffuse astrocytoma grade II (10-y PFS, 0.65 vs 0.70). This supports the recent analysis by Merchant et al72 (0.64 at 10 y). Mishra et al73 found 10-year PFS of 0.52 in patients treated between 1970 and 1995. The specific aspect of unsuccessful radiation treatment for disseminated tumors is detailed elsewhere.33 NF-1 patients had not been excluded from radiotherapy, as they would be today to avoid the undesirable effects on neuropsychological development72,77 or vascular integrity.79,100,101 Fatal moyamoya occurred 3 years postradiotherapy in 1 non–NF-1 patient, who had a diagnosis of a hypothalamic-chiasmatic PA at age 4 years and received chemotherapy at age 5 years and radiotherapy at age 10 years. So far, we lack further information on the long-term sequelae of our radiotherapy group.
In comprehensive LGG strategies, the role of modern radiation therapy has to be redefined to preserve functionally relevant areas within the brain and avoid adverse side effects.47
Overall Survival
OS was 0.94 in this prospectively followed cohort from a multicenter study, with a median follow-up of almost 10 years. In larger, retrospective series, OS ranged from 83% at 10 years (63% of children observed, 32% radiotherapy, 5% chemotherapy)37 to 97.6% and 94.6% at 10 years for observed and treated patients.39 Despite unsatisfactory progression rates in subgroups, OS remained high in the treatment arms (Table 3). In the recent COG trial series, only NF-1 status was positively predictive for OS in children treated with chemotherapy.43 In the British series,34 patients aged <1 year had less favorable survival, but possible other influential variables were not addressed. We stress the relevance of age and add DS and incomplete resection as unfavorable factors after multivariate analysis. On the other hand, surgical morbidity should always be kept in mind,102,103 especially considering that at most sites even subtotal resection does not seem to be advantageous.
We are the first group to report inferior survival for children aged >11 years. These patients often have grade II gliomas (13.3%), and the negative effects of these tumors have variably been related to location, resection, or biologic properties.37,52,58,104,105 In our study, however, only 5 patients died of progressive grade II tumors, whereas 8 had grade I, and 4/17 deaths in this age group were not related to progression. Larger trials than ours will be needed to elucidate the role of age.
Recent retrospective reviews, spanning some decades, have demonstrated that progression of pediatric LGGs following initial surgical intervention can continue well beyond 10 years,36,37 and thus survival analysis requires prolonged follow-up.
In conclusion, children with LGG merit comprehensive, multidisciplinary treatment strategies with a focus on age, NF-1 status, tumor site, histology, and extent of resection. The HIT-LGG-1996 algorithm has met this need. A nationwide multimodal treatment strategy was feasible and broadly accepted for pediatric LGG patients, and extended follow-up yielded results for OS, EFS, and PFS comparable to those of single-institution series. Specifically, the start of radiotherapy was deferred by a vincristine-carboplatin chemotherapy approach for the majority of those needing nonsurgical treatment. Evidence of the efficacy of chemotherapy for saving vision in the young has yet to be produced.88
We identified patients aged <1 year or with DS or dissemination as a high-risk group with poor prognosis. These patients need early treatment for severe symptoms and/or progression, as well as multiple treatments due to repeated progression. To meet their demands, future trials need risk-adapted strategies, considering conventional89,106 as well as new approaches.107,108 Information on the molecular signature of LGGs is imperative,109,110 since different genetic changes may drive their behavior, as has been recently shown for childhood medulloblastoma.111,112
Funding
The HIT-LGG-1996 study was supported by grant no. 70-2218-Gn1 from Deutsche Krebshilfe.
Acknowledgments
We acknowledge the solid data management and secretarial assistance of Sylvia Soellner, Marina Geh, and Sabine Breitmoser-Greiner. We thank all members of the trial commission and the pediatric oncology teams of the participating hospitals for their support and cooperation (see detailed list23).
Conflict of interest statement. None declared.
References
- 1.Abdollahzadeh M, Hoffman HJ, Blazer SI, et al. Benign cerebellar astrocytoma in childhood: experience at the Hospital for Sick Children 1980–1992. Childs Nerv Syst. 1994;10(6):380–383. doi: 10.1007/BF00335126. [DOI] [PubMed] [Google Scholar]
- 2.Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Campbell JW, Pollack IF. Cerebellar astrocytomas in children. J Neurooncol. 1996;28(2–3):223–231. doi: 10.1007/BF00250201. [DOI] [PubMed] [Google Scholar]
- 4.Constantini S, Houten J, Miller DC, et al. Intramedullary spinal cord tumors in children under the age of 3 years. J Neurosurg. 1996;85(6):1036–1043. doi: 10.3171/jns.1996.85.6.1036. [DOI] [PubMed] [Google Scholar]
- 5.Pollack IF, Claassen D, al-Shboul Q, Janosky JE, Deutsch M. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82(4):536–547. doi: 10.3171/jns.1995.82.4.0536. [DOI] [PubMed] [Google Scholar]
- 6.Sutton LN, Molloy PT, Sernyak H, et al. Long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. J Neurosurg. 1995;83(4):583–589. doi: 10.3171/jns.1995.83.4.0583. [DOI] [PubMed] [Google Scholar]
- 7.Wisoff JH, Abbott R, Epstein F. Surgical management of exophytic chiasmatic-hypothalamic tumors of childhood. J Neurosurg. 1990;73(5):661–667. doi: 10.3171/jns.1990.73.5.0661. [DOI] [PubMed] [Google Scholar]
- 8.Wisoff JH, Sanford RA, Heier LA, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery. 2011;68(6):1548–1554. doi: 10.1227/NEU.0b013e318214a66e. discussion 1554–1555. [DOI] [PubMed] [Google Scholar]
- 9.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15(8):2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 10.Gaynon PS, Ettinger LJ, Baum ES, Siegel SE, Krailo MD, Hammond GD. Carboplatin in childhood brain tumors. A Children's Cancer Study Group phase II trial. Cancer. 1990;66(12):2465–2469. doi: 10.1002/1097-0142(19901215)66:12<2465::aid-cncr2820661204>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Packer RJ, Lange B, Ater J, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11(5):850–856. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- 12.Grabb PA, Lunsford LD, Albright AL, Kondziolka D, Flickinger JC. Stereotactic radiosurgery for glial neoplasms of childhood. Neurosurgery. 1996;38(4):696–701. [PubMed] [Google Scholar]
- 13.Pierce SM, Barnes PD, Loeffler JS, McGinn C, Tarbell NJ. Definitive radiation therapy in the management of symptomatic patients with optic glioma. Survival and long-term effects. Cancer. 1990;65(1):45–52. doi: 10.1002/1097-0142(19900101)65:1<45::aid-cncr2820650111>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Tarbell NJ, Loeffler JS. Recent trends in the radiotherapy of pediatric gliomas. J Neurooncol. 1996;28(2–3):233–244. doi: 10.1007/BF00250202. [DOI] [PubMed] [Google Scholar]
- 15.West CG, Gattamaneni R, Blair V. Radiotherapy in the treatment of low-grade astrocytomas. I. A survival analysis. Childs Nerv Syst. 1995;11(8):438–442. doi: 10.1007/BF00334960. [DOI] [PubMed] [Google Scholar]
- 16.Bouffet E, Pierre-Kahn A, Marchal JC, et al. Prognostic factors in pediatric spinal cord astrocytoma. Cancer. 1998;83(11):2391–2399. doi: 10.1002/(sici)1097-0142(19981201)83:11<2391::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Dirven CM, Mooij JJ, Molenaar WM. Cerebellar pilocytic astrocytoma: a treatment protocol based upon analysis of 73 cases and a review of the literature. Childs Nerv Syst. 1997;13(1):17–23. doi: 10.1007/s003810050033. [DOI] [PubMed] [Google Scholar]
- 18.Smoots DW, Geyer JR, Lieberman DM, Berger MS. Predicting disease progression in childhood cerebellar astrocytoma. Childs Nerv Syst. 1998;14(11):636–648. doi: 10.1007/s003810050290. [DOI] [PubMed] [Google Scholar]
- 19.Jahraus CD, Tarbell NJ. Optic pathway gliomas. Pediatr Blood Cancer. 2006;46(5):586–596. doi: 10.1002/pbc.20655. [DOI] [PubMed] [Google Scholar]
- 20.Wiestler OD, Wolf HK. [Revised WHO classification and new developments in diagnosis of central nervous system tumors] [in German] Pathologe. 1995;16(4):245–255. doi: 10.1007/s002920050098. [DOI] [PubMed] [Google Scholar]
- 21.Kleihues P, Cavenee W. Pathology and Genetics of Tumors of the Nervous System. Lyon: International Agency for Research on Cancer (IARC) Press; 2000. [Google Scholar]
- 22.Walker D, Gnekow A, Perilongo G, Zanetti I. Vincristine (VCR) carboplatin (CBDCA) in hypothalamic-chiasmatic glioma (HCG): a report of the International Consortium on Low Grade Glioma (ICLGG) [abstract] Med Ped Oncol. 2002;39:229. [Google Scholar]
- 23.Gnekow AK, Kortmann RD, Pietsch T, Emser A. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy—report from the multicenter treatment study for children and adolescents with a low grade glioma—HIT-LGG 1996—of the Society of Pediatric Oncology and Hematology (GPOH) Klin Padiatr. 2004;216(6):331–342. doi: 10.1055/s-2004-832355. [DOI] [PubMed] [Google Scholar]
- 24.Peraud A, Goetz C, Siefert A, Tonn JC, Kreth FW. Interstitial iodine-125 radiosurgery alone or in combination with microsurgery for pediatric patients with eloquently located low-grade glioma: a pilot study. Childs Nerv Syst. 2007;23(1):39–46. doi: 10.1007/s00381-006-0203-7. [DOI] [PubMed] [Google Scholar]
- 25.Korinthenberg R, Neuburger D, Trippel M, Ostertag C, Nikkhah G. Long-term results of brachytherapy with temporary iodine-125 seeds in children with low-grade gliomas. Int J Radiat Oncol Biol Phys. 2011;79(4):1131–1138. doi: 10.1016/j.ijrobp.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan E, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Klein J. Small sample moments of the estimator of the variance of the Kaplan–Meier and Nelson–Aalen estimators. Scand J Stat. 1991;18:333–340. [Google Scholar]
- 28.Miller A. Selection of subsets of regression variables (with discussion) J Royal Stat Soc. Series A. 1984;147:389–425. [Google Scholar]
- 29.Gnekow AK. Recommendations of the Brain Tumor Subcommittee for the reporting of trials. SIOP Brain Tumor Subcommittee. International Society of Pediatric Oncology. Med Pediatr Oncol. 1995;24(2):104–108. doi: 10.1002/mpo.2950240209. [DOI] [PubMed] [Google Scholar]
- 30.Poussaint TY, Barnes PD, Nichols K, et al. Diencephalic syndrome: clinical features and imaging findings. AJNR Am J Neuroradiol. 1997;18(8):1499–1505. [PMC free article] [PubMed] [Google Scholar]
- 31.Gropman AL, Packer RJ, Nicholson HS, et al. Treatment of diencephalic syndrome with chemotherapy: growth, tumor response, and long term control. Cancer. 1998;83(1):166–172. doi: 10.1002/(sici)1097-0142(19980701)83:1<166::aid-cncr22>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Perilongo G, Carollo C, Salviati L, et al. Diencephalic syndrome and disseminated juvenile pilocytic astrocytomas of the hypothalamic-optic chiasm region. Cancer. 1997;80(1):142–146. doi: 10.1002/(sici)1097-0142(19970701)80:1<142::aid-cncr19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.von Hornstein S, Kortmann RD, Pietsch T, et al. Impact of chemotherapy on disseminated low-grade glioma in children and adolescents: report from the HIT-LGG 1996 trial. Pediatr Blood Cancer. 2011;56(7):1046–1054. doi: 10.1002/pbc.23006. [DOI] [PubMed] [Google Scholar]
- 34.Stokland T, Liu JF, Ironside JW, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702) Neuro Oncol. 2010;12(12):1257–1268. doi: 10.1093/neuonc/noq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher BJ, Leighton CC, Vujovic O, Macdonald DR, Stitt L. Results of a policy of surveillance alone after surgical management of pediatric low grade gliomas. Int J Radiat Oncol Biol Phys. 2001;51(3):704–710. doi: 10.1016/s0360-3016(01)01705-9. [DOI] [PubMed] [Google Scholar]
- 36.Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;115(24):5761–5770. doi: 10.1002/cncr.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51(2):245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 38.Hernaiz Driever P, von Hornstein S, Pietsch T, et al. Natural history and management of low-grade glioma in NF-1 children. J Neurooncol. 2010;100(2):199–207. doi: 10.1007/s11060-010-0159-z. [DOI] [PubMed] [Google Scholar]
- 39.Nicolin G, Parkin P, Mabbott D, et al. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer. 2009;53(7):1231–1237. doi: 10.1002/pbc.22198. [DOI] [PubMed] [Google Scholar]
- 40.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 41.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20(20):4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 42.Laithier V, Grill J, Le Deley MC, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy—results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21(24):4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 43.Ater J, Holmes E, Zhou T, et al. Results of COG protocol A9952: a randomized phase 3 study of two chemotherapy regimes for incompletely resected low-grade glioma in young children. Neuro Oncol. 2008;10(3):451. [Google Scholar]
- 44.Adriaensen ME, Schaefer-Prokop CM, Stijnen T, Duyndam DA, Zonnenberg BA, Prokop M. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol. 2009;16(6):691–696. doi: 10.1111/j.1468-1331.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 45.Fletcher WA, Imes RK, Hoyt WF. Chiasmal gliomas: appearance and long-term changes demonstrated by computerized tomography. J Neurosurg. 1986;65(2):154–159. doi: 10.3171/jns.1986.65.2.0154. [DOI] [PubMed] [Google Scholar]
- 46.Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg Spine. 2000;93(2):183–193. doi: 10.3171/spi.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 47.Scheinemann K, Bartels U, Huang A, et al. Survival and functional outcome of childhood spinal cord low-grade gliomas. Clinical article. J Neurosurg Pediatr. 2009;4(3):254–261. doi: 10.3171/2009.4.PEDS08411. [DOI] [PubMed] [Google Scholar]
- 48.Stiller CA, Nectoux J. International incidence of childhood brain and spinal tumours. Int J Epidemiol. 1994;23(3):458–464. doi: 10.1093/ije/23.3.458. [DOI] [PubMed] [Google Scholar]
- 49.Watson GA, Kadota RP, Wisoff JH. Multidisciplinary management of pediatric low-grade gliomas. Semin Radiat Oncol. 2001;11(2):152–162. doi: 10.1053/srao.2001.21421. [DOI] [PubMed] [Google Scholar]
- 50.Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999;58(10):1061–1068. doi: 10.1097/00005072-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225. doi: 10.1093/jnen/61.3.215. discussion 226–229. [DOI] [PubMed] [Google Scholar]
- 52.Jones DT, Mulholland SA, Pearson DM, et al. Adult grade II diffuse astrocytomas are genetically distinct from and more aggressive than their paediatric counterparts. Acta Neuropathol. 2011;121(6):753–761. doi: 10.1007/s00401-011-0810-6. [DOI] [PubMed] [Google Scholar]
- 53.Dirks PB, Jay V, Becker LE, et al. Development of anaplastic changes in low-grade astrocytomas of childhood. Neurosurgery. 1994;34(1):68–78. [PubMed] [Google Scholar]
- 54.Krieger MD, Gonzalez-Gomez I, Levy ML, McComb JG. Recurrence patterns and anaplastic change in a long-term study of pilocytic astrocytomas. Pediatr Neurosurg. 1997;27(1):1–11. doi: 10.1159/000121218. [DOI] [PubMed] [Google Scholar]
- 55.Zoeller GK, Brathwaite CD, Sandberg DI. Malignant transformation of an optic pathway glioma without prior radiation therapy. J Neurosurg Pediatr. 2010;5(5):507–510. doi: 10.3171/2009.12.PEDS09173. [DOI] [PubMed] [Google Scholar]
- 56.Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25(6):682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 57.Massimino M, Spreafico F, Riva D, et al. A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol. 2010;100(1):65–71. doi: 10.1007/s11060-010-0136-6. [DOI] [PubMed] [Google Scholar]
- 58.Pollack IF, Hamilton RL, Sobol RW, et al. IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children's Oncology Group. Childs Nerv Syst. 2010;27(1):87–94. doi: 10.1007/s00381-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warmuth-Metz M, Bison B, Leykamm S. Neuroradiologic review in pediatric brain tumor studies. Klin Neuroradiol. 2009;19(4):263–273. doi: 10.1007/s00062-009-9029-5. [DOI] [PubMed] [Google Scholar]
- 60.Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75(4):1051–1059. doi: 10.1002/1097-0142(19950215)75:4<1051::aid-cncr2820750423>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 61.Leonard JR, Perry A, Rubin JB, King AA, Chicoine MR, Gutmann DH. The role of surgical biopsy in the diagnosis of glioma in individuals with neurofibromatosis-1. Neurology. 2006;67(8):1509–1512. doi: 10.1212/01.wnl.0000240076.31298.47. [DOI] [PubMed] [Google Scholar]
- 62.Ekinci G, Akpinar IN, Baltacioglu F, et al. Early-postoperative magnetic resonance imaging in glial tumors: prediction of tumor regrowth and recurrence. Eur J Radiol. 2003;45(2):99–107. doi: 10.1016/s0720-048x(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 63.Pencalet P, Maixner W, Sainte-Rose C, et al. Benign cerebellar astrocytomas in children. J Neurosurg. 1999;90(2):265–273. doi: 10.3171/jns.1999.90.2.0265. [DOI] [PubMed] [Google Scholar]
- 64.Merchant TE, Kiehna EN, Thompson SJ, Heideman R, Sanford RA, Kun LE. Pediatric low-grade and ependymal spinal cord tumors. Pediatr Neurosurg. 2000;32(1):30–36. doi: 10.1159/000028894. [DOI] [PubMed] [Google Scholar]
- 65.Silva MM, Goldman S, Keating G, Marymont MA, Kalapurakal J, Tomita T. Optic pathway hypothalamic gliomas in children under three years of age: the role of chemotherapy. Pediatr Neurosurg. 2000;33(3):151–158. doi: 10.1159/000028996. [DOI] [PubMed] [Google Scholar]
- 66.Souweidane MM, Hoffman HJ. Current treatment of thalamic gliomas in children. J Neurooncol. 1996;28(2–3):157–166. doi: 10.1007/BF00250196. [DOI] [PubMed] [Google Scholar]
- 67.Grill J, Laithier V, Rodriguez D, Raquin MA, Pierre-Kahn A, Kalifa C. When do children with optic pathway tumours need treatment? An oncological perspective in 106 patients treated in a single centre. Eur J Pediatr. 2000;159(9):692–696. doi: 10.1007/s004310000531. [DOI] [PubMed] [Google Scholar]
- 68.Huber J, Sovinz P, Lackner H, Mokry M, Eder H, Urban C. Diencephalic syndrome: a frequently delayed diagnosis in failure to thrive. Klin Padiatr. 2007;219(2):91–94. doi: 10.1055/s-2007-921559. [DOI] [PubMed] [Google Scholar]
- 69.Shaw EG, Wisoff JH. Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro Oncol. 2003;5(3):153–160. doi: 10.1215/S1152-8517-02-00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanford A, Kun L, Sposto R, et al. Low-grade gliomas of childhood: impact of surgical resection. A report from the Childreńs Oncology Group [abstract] J Neurosurg. 2002;96:427–428. [Google Scholar]
- 71.Scheinemann K, Bartels U, Tsangaris E, et al. Feasibility and efficacy of repeated chemotherapy for progressive pediatric low-grade gliomas. Pediatr Blood Cancer. 2011;57(1):84–88. doi: 10.1002/pbc.22917. [DOI] [PubMed] [Google Scholar]
- 72.Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27(22):3598–3604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mishra KK, Puri DR, Missett BT, et al. The role of up-front radiation therapy for incompletely resected pediatric WHO grade II low-grade gliomas. Neuro Oncol. 2006;8(2):166–174. doi: 10.1215/15228517-2005-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32(3):235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 75.Packer RJ, Meadows AT, Rorke LB, Goldwein JL, D'Angio G. Long-term sequelae of cancer treatment on the central nervous system in childhood. Med Pediatr Oncol. 1987;15(5):241–253. doi: 10.1002/mpo.2950150505. [DOI] [PubMed] [Google Scholar]
- 76.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–3697. doi: 10.1200/JCO.2008.21.2738. Epub 2009 Jul 3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aarsen FK, Paquier PF, Arts WF, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol. 2009;27(21):3526–3532. doi: 10.1200/JCO.2008.19.6303. Epub 2009 May 3511. [DOI] [PubMed] [Google Scholar]
- 78.Lacaze E, Kieffer V, Streri A, et al. Neuropsychological outcome in children with optic pathway tumours when first-line treatment is chemotherapy. Br J Cancer. 2003;89(11):2038–2044. doi: 10.1038/sj.bjc.6601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tacke U, Karger D, Spreer J, Berlis A, Nikkhah G, Korinthenberg R. Incidence of vasculopathy in children with hypothalamic/chiasmatic gliomas treated with brachytherapy. Childs Nerv Syst. 2011;27(6):961–966. doi: 10.1007/s00381-010-1370-0. [DOI] [PubMed] [Google Scholar]
- 80.Mahoney DH, Jr., Cohen ME, Friedman HS, et al. Carboplatin is effective therapy for young children with progressive optic pathway tumors: a Pediatric Oncology Group phase II study. Neuro Oncol. 2000;2(4):213–220. doi: 10.1093/neuonc/2.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marcus KJ, Goumnerova L, Billett AL, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61(2):374–379. doi: 10.1016/j.ijrobp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 82.Kortmann RD, Timmermann B, Taylor RE, et al. Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part I: Treatment modalities of radiation therapy. Strahlenther Onkol. 2003;179(8):509–520. doi: 10.1007/s00066-003-9104-9. [DOI] [PubMed] [Google Scholar]
- 83.Combs SE, Schulz-Ertner D, Moschos D, Thilmann C, Huber PE, Debus J. Fractionated stereotactic radiotherapy of optic pathway gliomas: tolerance and long-term outcome. Int J Radiat Oncol Biol Phys. 2005;62(3):814–819. doi: 10.1016/j.ijrobp.2004.12.081. [DOI] [PubMed] [Google Scholar]
- 84.Lafay-Cousin L, Sung L, Carret AS, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma: a Canadian Pediatric Brain Tumor Consortium experience. Cancer. 2008;112(4):892–899. doi: 10.1002/cncr.23249. [DOI] [PubMed] [Google Scholar]
- 85.Genc DB, Canpolat C, Berrak SG. Clinical features and management of carboplatin-related hypersensitivity reactions in pediatric low-grade glioma. Support Care Cancer. 2012;20:385–393. doi: 10.1007/s00520-011-1123-y. [DOI] [PubMed] [Google Scholar]
- 86.Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol. 2007;9(4):430–437. doi: 10.1215/15228517-2007-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campagna M, Opocher E, Viscardi E, et al. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer. 2010;55(6):1083–1088. doi: 10.1002/pbc.22748. [DOI] [PubMed] [Google Scholar]
- 88.Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010;46(12):2253–2259. doi: 10.1016/j.ejca.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 89.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52(7):791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 90.Ronghe M, Hargrave D, Bartels U, et al. Vincristine and carboplatin chemotherapy for unresectable and/or recurrent low-grade astrocytoma of the brainstem. Pediatr Blood Cancer. 2010;55(3):471–477. doi: 10.1002/pbc.22557. [DOI] [PubMed] [Google Scholar]
- 91.Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42(12):1807–1816. doi: 10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 92.Perilongo G, Garre ML, Giangaspero F. Low-grade gliomas and leptomeningeal dissemination: a poorly understood phenomenon. Childs Nerv Syst. 2003;19(4):197–203. doi: 10.1007/s00381-003-0733-1. [DOI] [PubMed] [Google Scholar]
- 93.Hukin J, Siffert J, Cohen H, Velasquez L, Zagzag D, Allen J. Leptomeningeal dissemination at diagnosis of pediatric low-grade neuroepithelial tumors. Neuro Oncol. 2003;5(3):188–196. doi: 10.1215/S1152-8517-02-00029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gajjar A, Bhargava R, Jenkins JJ, et al. Low-grade astrocytoma with neuraxis dissemination at diagnosis. J Neurosurg. 1995;83(1):67–71. doi: 10.3171/jns.1995.83.1.0067. [DOI] [PubMed] [Google Scholar]
- 95.Pollack IF, Hurtt M, Pang D, Albright AL. Dissemination of low grade intracranial astrocytomas in children. Cancer. 1994;73(11):2869–2878. doi: 10.1002/1097-0142(19940601)73:11<2869::aid-cncr2820731134>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 96.Kovalic JJ, Grigsby PW, Shepard MJ, Fineberg BB, Thomas PR. Radiation therapy for gliomas of the optic nerve and chiasm. Int J Radiat Oncol Biol Phys. 1990;18(4):927–932. doi: 10.1016/0360-3016(90)90418-j. [DOI] [PubMed] [Google Scholar]
- 97.Grabenbauer GG, Schuchardt U, Buchfelder M, et al. Radiation therapy of optico-hypothalamic gliomas (OHG)—radiographic response, vision and late toxicity. Radiother Oncol. 2000;54(3):239–245. doi: 10.1016/s0167-8140(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 98.Saran FH, Baumert BG, Khoo VS, et al. Stereotactically guided conformal radiotherapy for progressive low-grade gliomas of childhood. Int J Radiat Oncol Biol Phys. 2002;53(1):43–51. doi: 10.1016/s0360-3016(02)02734-7. [DOI] [PubMed] [Google Scholar]
- 99.Erkal HS, Serin M, Cakmak A. Management of optic pathway and chiasmatic-hypothalamic gliomas in children with radiation therapy. Radiother Oncol. 1997;45(1):11–15. doi: 10.1016/s0167-8140(97)00102-3. [DOI] [PubMed] [Google Scholar]
- 100.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45(3):393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 101.Horn P, Pfister S, Bueltmann E, Vajkoczy P, Schmiedek P. Moyamoya-like vasculopathy (moyamoya syndrome) in children. Childs Nerv Syst. 2004;20(6):382–391. doi: 10.1007/s00381-004-0960-0. [DOI] [PubMed] [Google Scholar]
- 102.Rueckriegel SM, Driever PH, Blankenburg F, Ludemann L, Henze G, Bruhn H. Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int J Radiat Oncol Biol Phys. 2010;76(3):859–866. doi: 10.1016/j.ijrobp.2009.02.054. [DOI] [PubMed] [Google Scholar]
- 103.Rueckriegel SM, Blankenburg F, Henze G, Baque H, Driever PH. Loss of fine motor function correlates with ataxia and decline of cognition in cerebellar tumor survivors. Pediatr Blood Cancer. 2009;53(3):424–431. doi: 10.1002/pbc.22104. [DOI] [PubMed] [Google Scholar]
- 104.Peters O, Gnekow AK, Rating D, Wolff JE. Impact of location on outcome in children with low-grade oligodendroglioma. Pediatr Blood Cancer. 2004;43(3):250–256. doi: 10.1002/pbc.20111. [DOI] [PubMed] [Google Scholar]
- 105.Hargrave D. Paediatric high and low grade glioma: the impact of tumour biology on current and future therapy. Br J Neurosurg. 2009;23(4):351–363. doi: 10.1080/02688690903158809. [DOI] [PubMed] [Google Scholar]
- 106.Cappellano AM, Bouffet E, Cavalheiro S, Seixas MT, da Silva NS. Diffuse intrinsic brainstem tumor in an infant: a case of therapeutic efficacy with vinorelbine. J Pediatr Hematol Oncol. 2011;33(2):116–118. doi: 10.1097/MPH.0b013e3181fe68e0. [DOI] [PubMed] [Google Scholar]
- 107.Jakacki RI, Hamilton M, Gilbertson RJ, et al. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children's Oncology Group Phase I Consortium Study. J Clin Oncol. 2008;26(30):4921–4927. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68(5):1520–1528. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
- 109.Jones DT, Kocialkowski S, Liu L, Pearson DM, Ichimura K, Collins VP. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28(20):2119–2123. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pfister S, Remke M, Benner A, et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27(10):1627–1636. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 112.Kool M, Koster J, Bunt J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]