Abstract
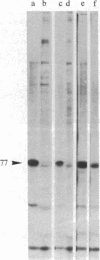
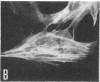
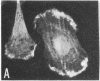
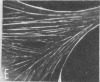
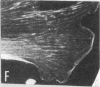
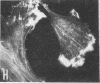
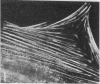
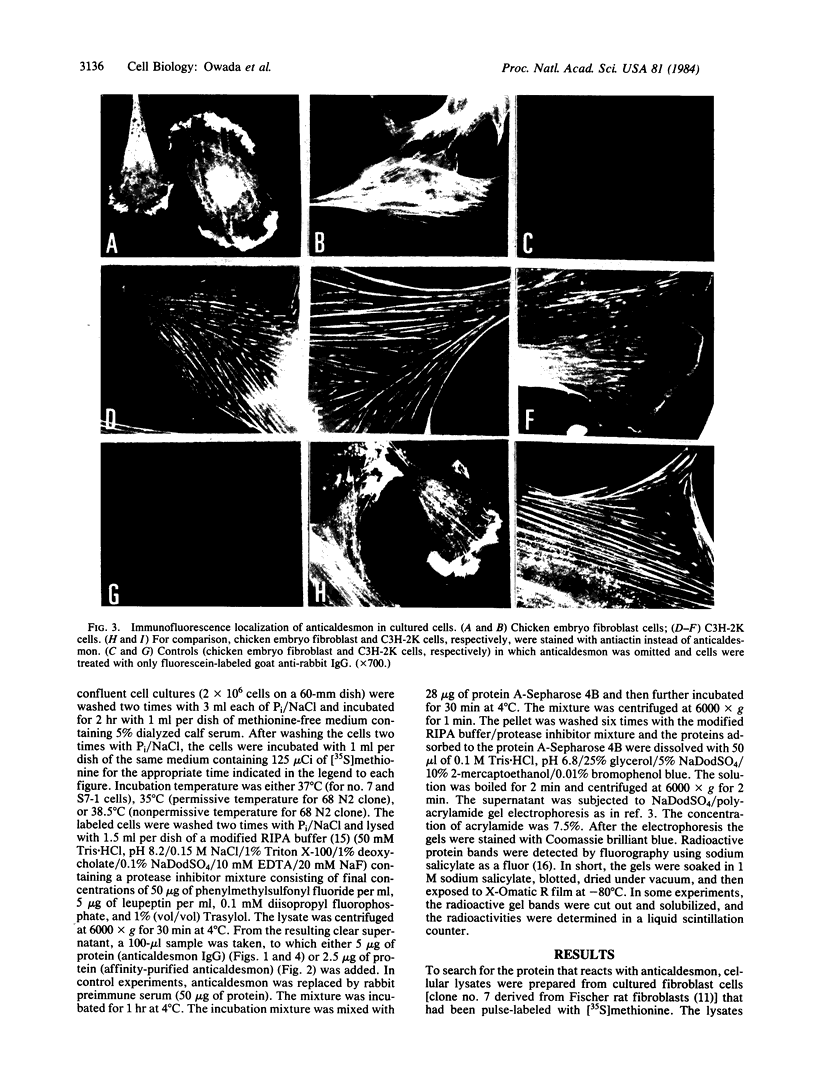
Caldesmon is a calmodulin-binding and F-actin-binding protein originally purified from chicken gizzard smooth muscle. This protein binds to F-actin filaments in a Ca2+- and calmodulin-dependent "flip-flop" fashion, thereby regulating the function of actin filaments. Here we report that various lines of cultured cells contain a Mr 77,000 protein that specifically reacts with the affinity-purified caldesmon antibody raised against chicken gizzard caldesmon . Among the fibroblast proteins that had been pulse-labeled with [35S]methionine, the Mr 77,000 protein was the only protein band detected on the NaDodSO4 gel that reacted with the anticaldesmon . The subcellular distribution of the Mr 77,000 protein was investigated by the indirect immunofluorescence technique using the anticaldesmon . In all fibroblast cell lines examined, the immunofluorescence localized along the cellular stress fibers and in leading edges of the cell. In Rous sarcoma virus-transformed cells (S7-1), however, the distribution of the fluorescence changed to a diffuse and blurred appearance. These staining patterns of anticaldesmon obtained with the normal and transformed cells coincided with those of antiactin in the corresponding states, strongly suggesting the functional linkage between the Mr 77,000 protein and actin filaments. We propose to refer to this Mr 77,000 protein as caldesmon 77. The cellular level of caldesmon 77 in transformed S7-1 cells decreased to about one-third of that in their normal counterparts (cell line no. 7). Essentially the same result was obtained with normal rat kidney cells infected with the temperature-sensitive transformation mutant Schmidt-Ruppin strain of Rous sarcoma virus (68 N2 clone). The cellular level of caldesmon 77 observed at a permissive temperature (35 degrees C) was about one-third of that at a nonpermissive temperature (38.5 degrees C). These changes of caldesmon 77 in transformed cells may correlate with the loss of Ca2+ regulation in the transformed state.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley R. A., Woods A., Smith C. G., Rees D. A. Actomyosin relationships with surface features in fibroblast adhesion. Exp Cell Res. 1980 Apr;126(2):263–272. doi: 10.1016/0014-4827(80)90264-5. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T., Takahashi S., Hayashi H., Hatano S. Fragmin: a calcium ion sensitive regulatory factor on the formation of actin filaments. Biochemistry. 1980 Jun 10;19(12):2677–2683. doi: 10.1021/bi00553a021. [DOI] [PubMed] [Google Scholar]
- Inoue H., Yutsudo M., Hakura A. Rat mutant cells showing temperature sensitivity for transformation by wild-type Moloney murine sarcoma virus. Virology. 1983 Feb;125(1):242–245. doi: 10.1016/0042-6822(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Kakiuchi R., Inui M., Morimoto K., Kanda K., Sobue K., Kakiuchi S. Caldesmon, a calmodulin-binding, F actin-interacting protein, is present in aorta, uterus and platelets. FEBS Lett. 1983 Apr 18;154(2):351–356. doi: 10.1016/0014-5793(83)80181-1. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Immunofluorescence studies on the structure of actin filaments in tissue culture cells. J Histochem Cytochem. 1975 Jul;23(7):507–528. doi: 10.1177/23.7.1095651. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura N., Asano A. Ca2+-sensitive gelation of actin filaments by a new protein factor. Nature. 1979 Nov 1;282(5734):44–48. doi: 10.1038/282044a0. [DOI] [PubMed] [Google Scholar]
- Owada M., Moelling K. Temperature-sensitive kinase activity associated with various mutants of avian sarcoma viruses which are temperature sensitive for transformation. Virology. 1980 Feb;101(1):157–168. doi: 10.1016/0042-6822(80)90492-4. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Craig S. W. Meta-vinculin--a vinculin-related protein with solubility properties of a membrane protein. Nature. 1982 Dec 9;300(5892):533–535. doi: 10.1038/300533a0. [DOI] [PubMed] [Google Scholar]
- Sobue K., Morimoto K., Kanda K., Maruyama K., Kakiuchi S. Reconstitution of Ca2+-sensitive gelation of actin filaments with filamin, caldesmon and calmodulin. FEBS Lett. 1982 Feb 22;138(2):289–292. doi: 10.1016/0014-5793(82)80463-8. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y. Induction of cell replication. Exp Cell Res. 1968 Oct;52(2):439–444. doi: 10.1016/0014-4827(68)90485-0. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P., Adelstein R. S., Feramisco J. R., Burridge K. Characterization of antibodies to smooth muscle myosin kinase and their use in localizing myosin kinase in nonmuscle cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4738–4742. doi: 10.1073/pnas.78.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]