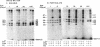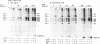Abstract
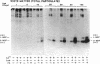
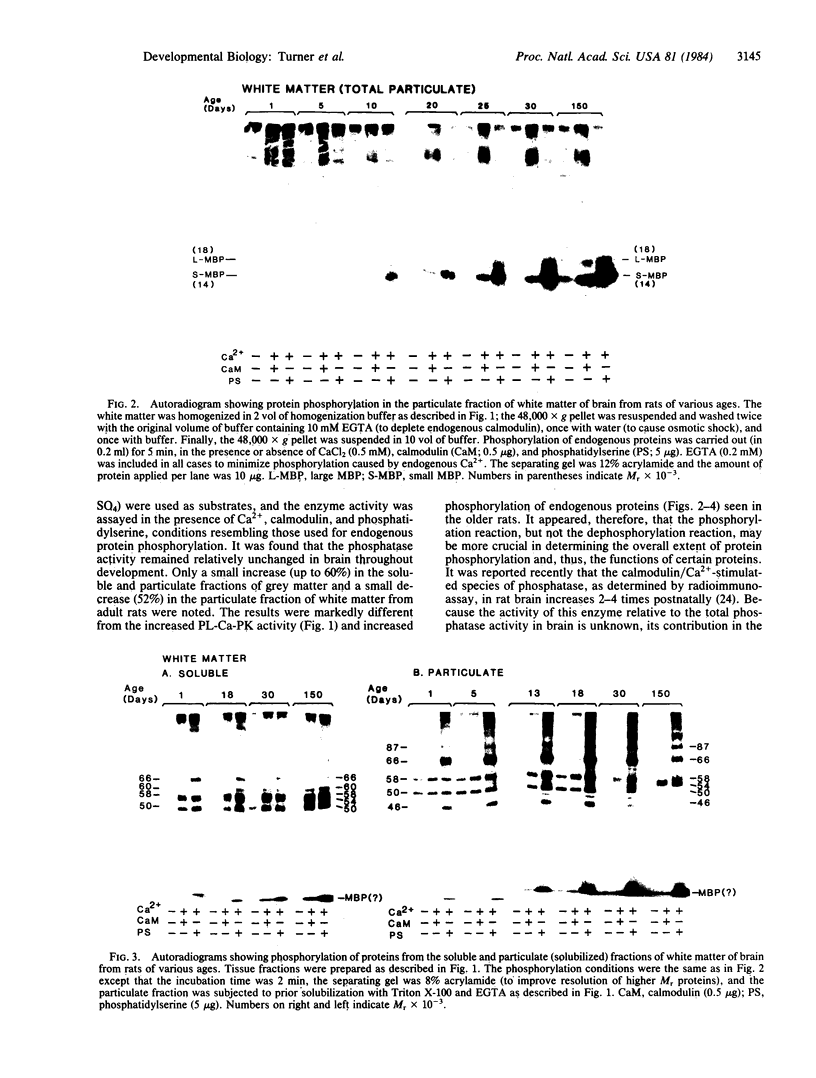
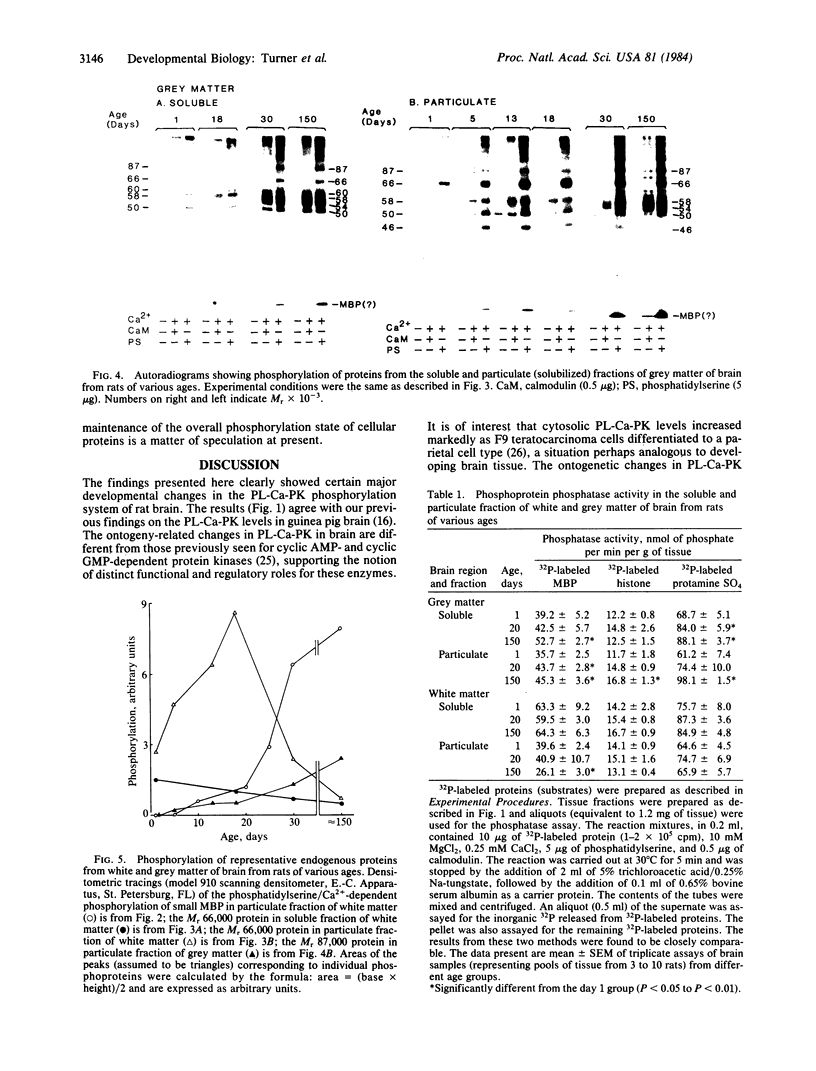
Ontogenetic changes in the protein phosphorylation/dephosphorylation systems in rat brain were investigated. It was found that the activity level of phospholipid-sensitive Ca2+-dependent protein kinase (PL-Ca-PK) in the particulate fraction of grey and white matter and the soluble fraction of grey matter increased rapidly and markedly after birth, reached the highest level at day 30, and declined slightly or remained unchanged thereafter. The enzyme level in the soluble fraction of white matter, in contrast, remained constant throughout the development and maturation of brain. Various ontogenetic changes in the substrate proteins for PL-Ca-PK were also noted. The levels of myelin basic protein and other substrates (notably the Mr 87,000, 58,000, 54,000, and 50,000 protein in grey matter) progressively increased during development, reaching the highest level at adulthood. The level of the Mr 66,000 protein from the particulate fraction of white and grey matter, on the other hand, increased rapidly after birth, reached a peak at day 18, and then declined to the initial neonatal level at the adult stage. The time scale for the increases in the levels of PL-Ca-PK and its many substrates paralleled that of brain development and maturation (synaptogenesis and myelinogenesis). The activity levels of phosphoprotein phosphatases (assayed using 32P-labeled myelin basic protein, histone, and protamine sulfate) were found to only slightly (up to 60%) increase or decrease in certain fractions from different brain regions during development, suggesting that phosphorylation, compared to dephosphorylation, may be more important in determining the phosphorylation state of cellular proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashendel C. L., Staller J. M., Boutwell R. K. Identification of a calcium- and phospholipid- dependent phorbol ester binding activity in the soluble fraction of mouse tissues. Biochem Biophys Res Commun. 1983 Feb 28;111(1):340–345. doi: 10.1016/s0006-291x(83)80157-0. [DOI] [PubMed] [Google Scholar]
- Bechtel P. J., Beavo J. A., Krebs E. G. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Apr 25;252(8):2691–2697. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Fiszman M. Y., Eppenberger H. M. Molecular and cell isoforms during development. Science. 1983 Sep 2;221(4614):921–927. doi: 10.1126/science.6348946. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Katoh N., Wise B. C., Kuo J. F. Phosphorylation of cardiac troponin inhibitory subunit (troponin I) and tropomyosin-binding subunit (troponin T) by cardiac phospholipid-sensitive Ca2+-dependent protein kinase. Biochem J. 1983 Jan 1;209(1):189–195. doi: 10.1042/bj2090189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh N., Wrenn R. W., Wise B. C., Shoji M., Kuo J. F. Substrate proteins for calmodulin-sensitive and phospholipid-sensitive Ca2+-dependent protein kinases in heart, and inhibition of their phosphorylation by palmitoylcarnitine. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4813–4817. doi: 10.1073/pnas.78.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Characterization of cytosolic calcium-activated phospholipid-dependent protein kinase activity in embryonal carcinoma cells. Effect of retinoc acid-induced differentiation of F9 cells to parietal endoderm. J Biol Chem. 1983 Aug 10;258(15):9178–9183. [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F. Changes in relative levels of guanosine-3':5'-monophosphate-dependent and adenosine-3':5'-monophosphate-dependent protein kinases in lung, heart, and brain of developing guinea pigs. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2256–2259. doi: 10.1073/pnas.72.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei G. J., Kuo J. F. Phosphorylation of skeletal-muscle troponin I and troponin T by phospholipid-sensitive Ca2+-dependent protein kinase and its inhibition by troponin C and tropomyosin. Biochem J. 1984 Mar 1;218(2):361–369. doi: 10.1042/bj2180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei G. J., Qi D. F., Schatzman R. C., Raynor R. L., Turner R. S., Kuo J. F. Comparative abilities of lanthanide ions La3+ and Tb3+ to substitute for Ca2+ in regulating phospholipid-sensitive Ca2+-dependent protein kinase and myosin light chain kinase. Life Sci. 1983 Jul 11;33(2):119–129. doi: 10.1016/0024-3205(83)90404-6. [DOI] [PubMed] [Google Scholar]
- Nagle D. S., Jaken S., Castagna M., Blumberg P. M. Variation with embryonic development and regional localization of specific [3H]phorbol 12,13-dibutyrate binding to brain. Cancer Res. 1981 Jan;41(1):89–93. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Schatzman R. C., Raynor R. L., Fritz R. B., Kuo J. F. Purification to homogeneity, characterization and monoclonal antibodies of phospholipid-sensitive Ca2+-dependent protein kinase from spleen. Biochem J. 1983 Feb 1;209(2):435–443. doi: 10.1042/bj2090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Calmodulin-dependent protein phosphatase: a developmental study. Biochemistry. 1983 Jul 19;22(15):3630–3635. doi: 10.1021/bi00284a014. [DOI] [PubMed] [Google Scholar]
- Turner R. S., Chou C. H., Kibler R. F., Kuo J. F. Basic protein in brain myelin is phosphorylated by endogenous phospholipid-sensitive Ca2+-dependent protein kinase. J Neurochem. 1982 Nov;39(5):1397–1404. doi: 10.1111/j.1471-4159.1982.tb12583.x. [DOI] [PubMed] [Google Scholar]
- Wise B. C., Andersson R. G., Mackerlova L., Raynor R. L., Solomonsson I., Kuo J. F. Ontogenetic aspects of phospholipid-sensitive calcium-dependent protein kinase in guinea pig tissues. Biochem Biophys Res Commun. 1981 Mar 31;99(2):407–413. doi: 10.1016/0006-291x(81)91760-5. [DOI] [PubMed] [Google Scholar]
- Wise B. C., Glass D. B., Chou C. H., Raynor R. L., Katoh N., Schatzman R. C., Turner R. S., Kibler R. F., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J Biol Chem. 1982 Jul 25;257(14):8489–8495. [PubMed] [Google Scholar]
- Wise B. C., Raynor R. L., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. I. Purification and general properties. J Biol Chem. 1982 Jul 25;257(14):8481–8488. [PubMed] [Google Scholar]
- Wrenn R. W., Katoh N., Schatzman R. C., Kuo J. F. Inhibition by phenothiazine antipsychotic drugs of calcium-dependent phosphorylation of cerebral cortex proteins regulated by phospholipid or calmodulin. Life Sci. 1981 Aug 17;29(7):725–733. doi: 10.1016/0024-3205(81)90026-6. [DOI] [PubMed] [Google Scholar]
- Wrenn R. W., Katoh N., Wise B. C., Kuo J. F. Stimulation by phosphatidylserine and calmodulin of calcium-dependent phosphorylation of endogenous proteins from cerebral cortex. J Biol Chem. 1980 Dec 25;255(24):12042–12046. [PubMed] [Google Scholar]