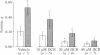Abstract
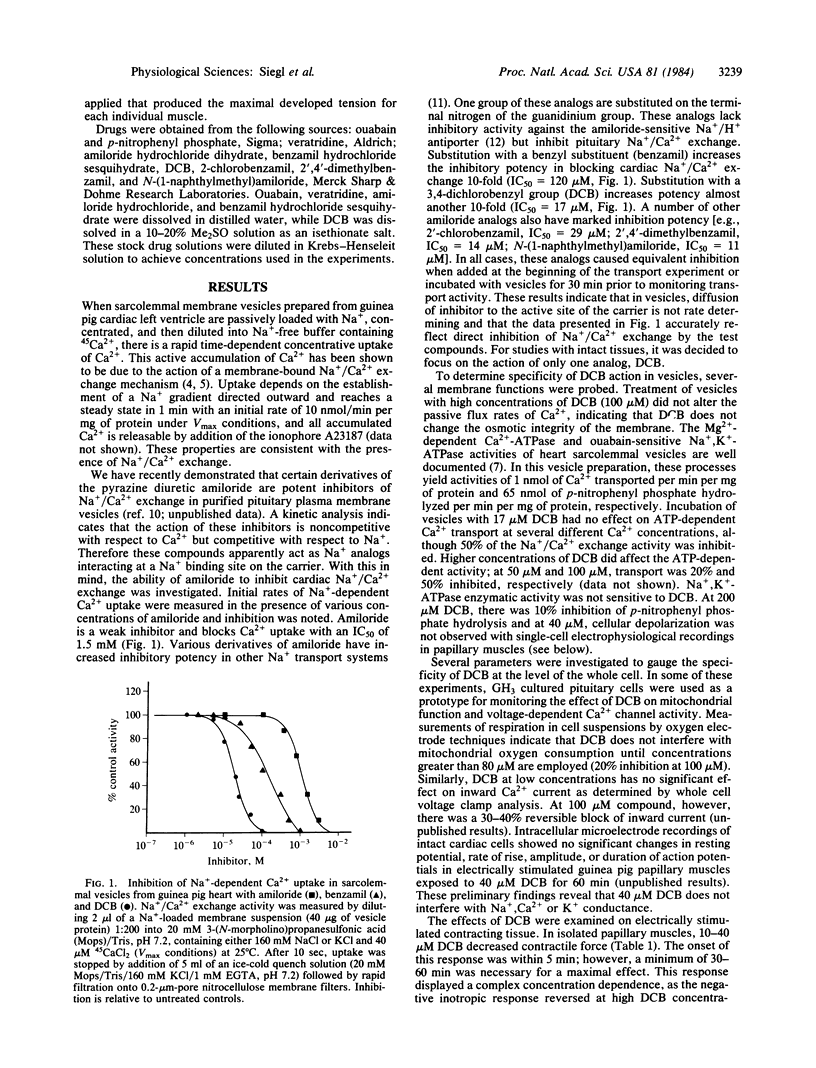
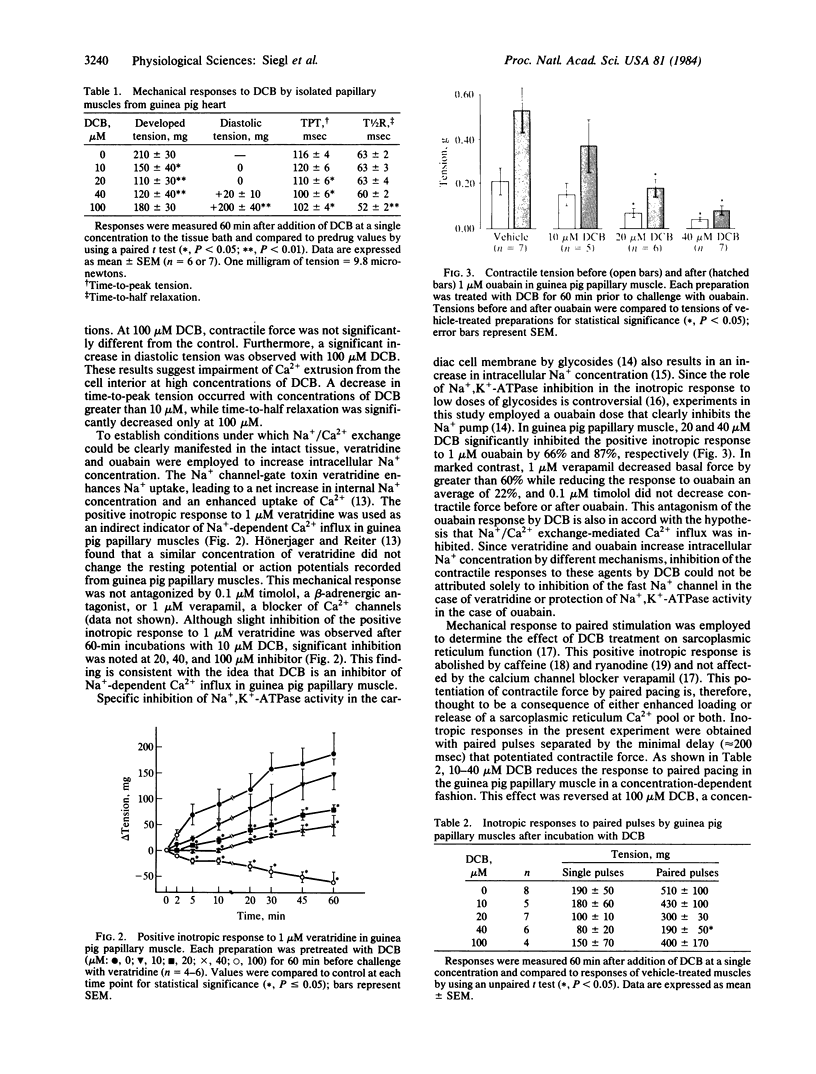
Na+/Ca2+ exchange is inhibited in both guinea pig cardiac membrane vesicles and papillary muscles in a concentration-dependent fashion by several analogs of the pyrazine diuretic amiloride. Structure/activity studies based on transport measurements in vesicles prepared from guinea pig left ventricle indicate that hydrophobic substitutions at the terminal nitrogen atom of the guanidinium moiety of amiloride improved the inhibitory potency almost 100-fold over that of the parent compound. 3',4'- Dichlorobenzamil ( DCB ) is one of the most active inhibitors (IC50 = 17 microM). In electrically stimulated papillary muscles isolated from guinea pig heart, 10-40 microM DCB decreases contractile force. At 100 microM inhibitor, diastolic tension is significantly increased. The positive inotropic responses to veratridine and ouabain are inhibited by 20 and 40 microM DCB . Since the responses to these interventions were a consequence of increased intracellular Na+ concentration, these data indicate that DCB is an inhibitor of Na+-dependent Ca2+ influx in the intact tissue. Interpretation of mechanical responses elicited by paired pulses suggests that 40 microM but not 100 microM DCB decreases release of Ca2+ from the sarcoplasmic reticulum. The mechanical data obtained with concentrations of DCB that inhibited Na+/Ca2+ exchange in vesicles suggest that a significant amount of Ca2+ can enter the cardiac cell via Na+/Ca2+ exchange under normal conditions and that this transport system may be an important source of Ca2+ supplying the sarcoplasmic reticulum in guinea pig heart. Moreover, these amiloride analogs function as potent inhibitors of the positive inotropic effect caused by increased intracellular Na+ concentration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Brody T. M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977 Sep;29(3):187–220. [PubMed] [Google Scholar]
- Barry W. H., Smith T. W. Mechanisms of transmembrane calcium movement in cultured chick embryo ventricular cells. J Physiol. 1982 Apr;325:243–260. doi: 10.1113/jphysiol.1982.sp014148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Colas B., Maroux S. Simultaneous isolation of brush border and basolateral membrane from rabbit enterocytes. Presence of brush border hydrolases in the basolateral membrane of rabbit enterocytes. Biochim Biophys Acta. 1980 Aug 4;600(2):406–420. doi: 10.1016/0005-2736(80)90444-7. [DOI] [PubMed] [Google Scholar]
- Daut J. The role of intracellular sodium ions in the regulation of cardiac contractility. J Mol Cell Cardiol. 1982 Mar;14(3):189–192. doi: 10.1016/0022-2828(82)90119-5. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Physiologie und Pharmakologie der transmembranären Natrium-, Kalium- und Calcium-Bewegungen. Arzneimittelforschung. 1972 Dec;22(12):2019–2028. [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. H., Brutsaert D. L., Forman R., Sonnenblick E. H. Influence of caffeine on force development and force-frequency relations in cat and rat heart muscle. Cardiovasc Res. 1974 Mar;8(2):162–172. doi: 10.1093/cvr/8.2.162. [DOI] [PubMed] [Google Scholar]
- Honerjäger P., Reiter M. The relation between the effects of veratridine on action potential and contraction in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(1):1–28. doi: 10.1007/BF00498026. [DOI] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Slow inward current and contraction in frog atrial muscle at various extracellular concentrations of Na and Ca ions. J Mol Cell Cardiol. 1979 Aug;11(8):733–753. doi: 10.1016/0022-2828(79)90400-0. [DOI] [PubMed] [Google Scholar]
- Kawata H., Ohba M., Hatae J., Kishi M. Paradoxical after-potentiation of the myocardial contractility by lanthanum. Jpn J Physiol. 1983;33(1):1–17. doi: 10.2170/jjphysiol.33.1. [DOI] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledvora R. F., Hegyvary C. Dependence of Na+-Ca2+ exchange and Ca2+-Ca2+ exchange on monovalent cations. Biochim Biophys Acta. 1983 Mar 23;729(1):123–136. doi: 10.1016/0005-2736(83)90463-7. [DOI] [PubMed] [Google Scholar]
- Michaelis M. L., Michaelis E. K. Alcohol and local anesthetic effects on Na+-dependent Ca2+ fluxes in brain synaptic membrane vesicles. Biochem Pharmacol. 1983 Mar 15;32(6):963–969. doi: 10.1016/0006-2952(83)90612-3. [DOI] [PubMed] [Google Scholar]
- Noble D. Mechanism of action of therapeutic levels of cardiac glycosides. Cardiovasc Res. 1980 Sep;14(9):495–514. doi: 10.1093/cvr/14.9.495. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Competitive interactions of sodium and calcium with the sodium-calcium exchange system of cardiac sarcolemmal vesicles. J Biol Chem. 1983 Mar 10;258(5):3178–3182. [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Sodium-calcium ion exchange in cardiac membrane vesicles. Proc Natl Acad Sci U S A. 1979 Feb;76(2):590–594. doi: 10.1073/pnas.76.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T., Templeton G. H., Jones L. R., Besch H. R., Jr Ryanodine: its alterations of cat papillary muscle contractile state and responsiveness to inotropic interventions and a suggested mechanism of action. J Pharmacol Exp Ther. 1979 Apr;209(1):37–47. [PubMed] [Google Scholar]
- Tillisch J. H., Fung L. K., Hom P. M., Langer G. A. Transient and steady-state effects of sodium and calcium on myocardial contractile response. J Mol Cell Cardiol. 1979 Feb;11(2):137–148. doi: 10.1016/0022-2828(79)90459-0. [DOI] [PubMed] [Google Scholar]
- Vigne P., Frelin C., Cragoe E. J., Jr, Lazdunski M. Ethylisopropyl-amiloride: a new and highly potent derivative of amiloride for the inhibition of the Na+/H+ exchange system in various cell types. Biochem Biophys Res Commun. 1983 Oct 14;116(1):86–90. doi: 10.1016/0006-291x(83)90384-4. [DOI] [PubMed] [Google Scholar]
- Wasserstrom J. A., Schwartz D. J., Fozzard H. A. Relation between intracellular sodium and twitch tension in sheep cardiac Purkinje strands exposed to cardiac glycosides. Circ Res. 1983 Jun;52(6):697–705. doi: 10.1161/01.res.52.6.697. [DOI] [PubMed] [Google Scholar]