Abstract
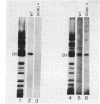
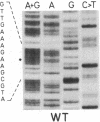
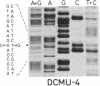
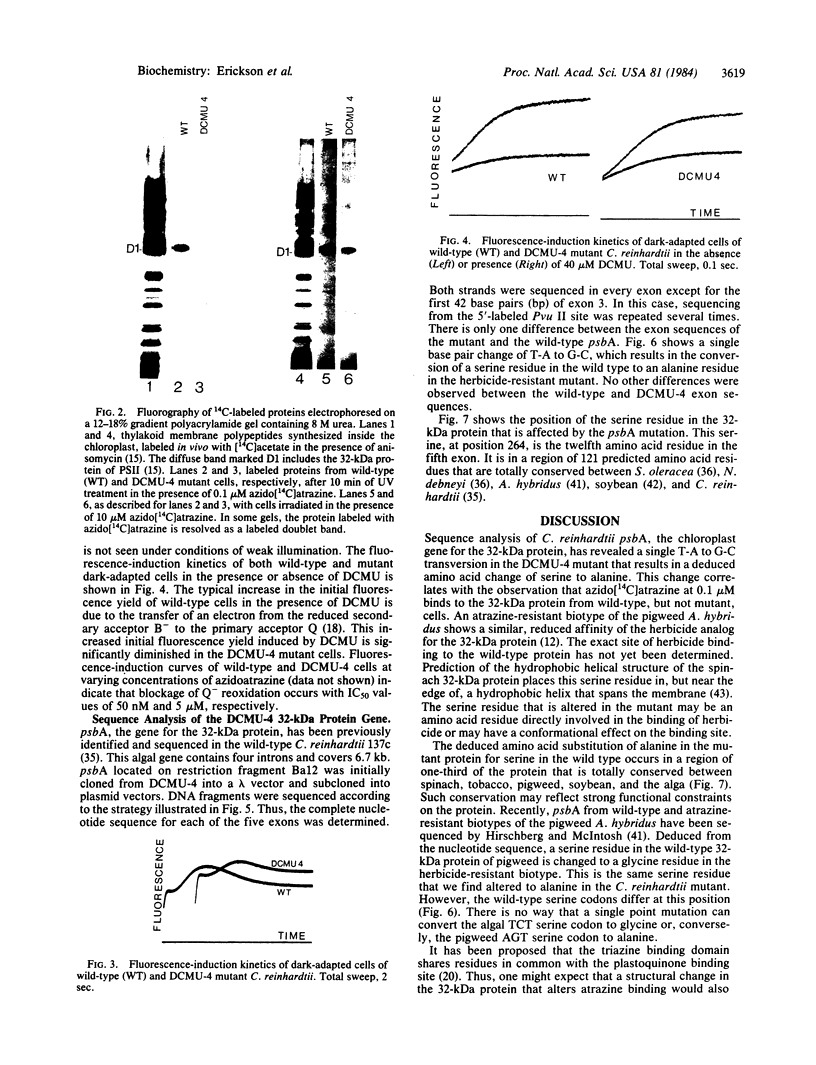
We report the isolation and characterization of a uniparental mutant of Chlamydomonas reinhardtii that is resistant to 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and 2-chloro-4-ethylamino-6-isopropylamino-s-triazine (atrazine). Such herbicides inhibit photosynthesis by preventing transfer of electrons in photosystem II from the primary stable electron acceptor Q to the secondary stable electron acceptor complex B, which is thought to contain a protein of 32 kDa and a bound quinone. It has been proposed that herbicide binding to the 32-kDa protein alters the B complex so that electron transfer from Q is prohibited. Both whole and broken-cell preparations of the mutant alga show a resistance to the effects of herbicide on electron transfer from Q to B, as measured by fluorescence-induction kinetics. In the absence of herbicide, mutant cells exhibit a slower rate of Q to B electron transfer than do wild-type cells. The 32-kDa protein from wild-type cells, but not mutant cells, binds azido[14C]atrazine at 0.1 μM. We have isolated psbA, the chloroplast gene for the 32-kDa protein, from both wild-type and herbicide-resistant algae and sequenced the coding regions of the gene that are contained in five exons. The only difference between the exon nucleotide sequences of the wild-type and mutant psbA is a single T-A to G-C transversion. This mutation results in a predicted amino acid change of serine in the wild-type protein to alanine in the mutant. We suggest that this alteration in the 32-kDa protein is the molecular basis for herbicide resistance in the C. reinhardtii mutant.
Keywords: psbA; 3-(3,4-dichlorophenyl)-1,1-dimethylurea; triazine herbicides; photosynthesis
Full text
PDF

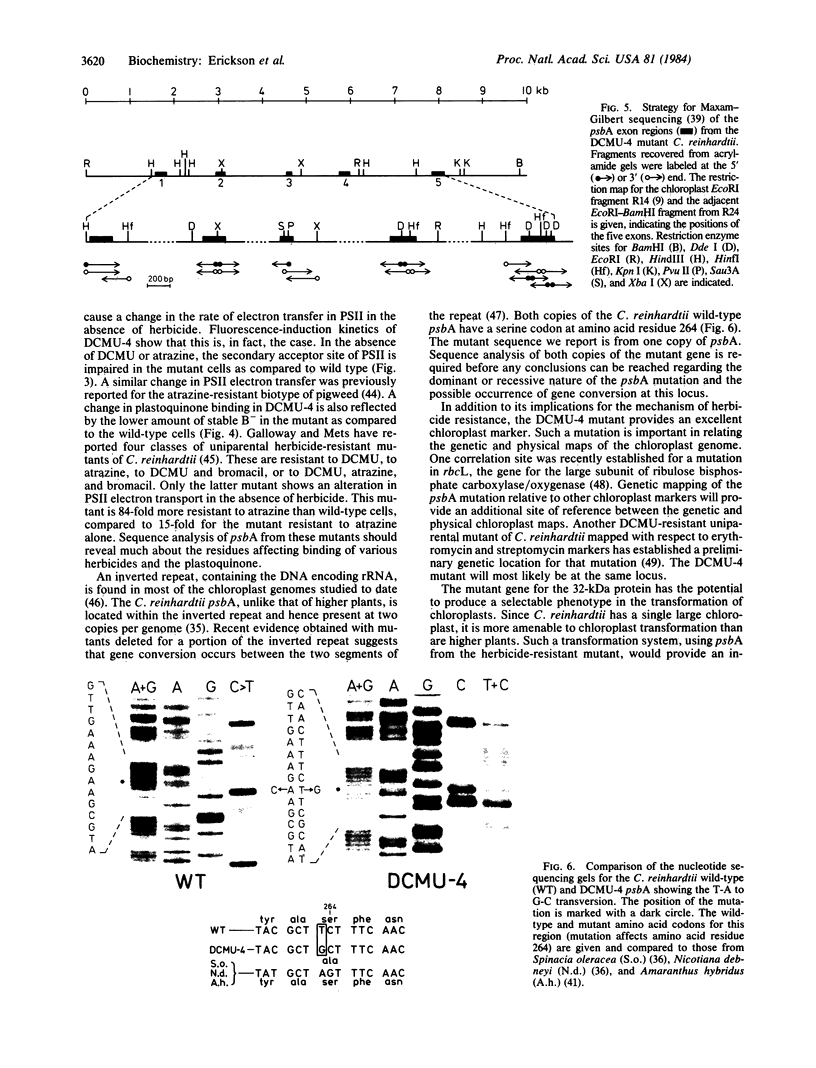

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennoun P. Correlation between states I and II in algae and the effect of magnesium on chloroplasts. Biochim Biophys Acta. 1974 Nov 19;368(2):141–147. doi: 10.1016/0005-2728(74)90144-3. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bowes J., Crofts A. R., Arntzen C. J. Redox Reactions on the reducing side of photosystem II in chloroplasts with altered herbicide binding properties. Arch Biochem Biophys. 1980 Apr 1;200(2):303–308. doi: 10.1016/0003-9861(80)90359-8. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Cervantes L., Covarrubias A., Soberón X., Vichido I., Blanco A., Kupersztoch-Portnoy Y. M., Bolivar F. Construction and characterization of new cloning vehicles. V. Mobilization and coding properties of pBR322 and several deletion derivatives including pBR327 and pBR328. Gene. 1981 Jan-Feb;13(1):25–35. doi: 10.1016/0378-1119(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D., Mets L. First DNA sequence of a chloroplast mutation: a missense alteration in the ribulosebisphosphate carboxylase large subunit gene. Plasmid. 1983 May;9(3):321–324. doi: 10.1016/0147-619x(83)90009-4. [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Rushford C. L., Dorney D. J., Wilson G. N., Schmickel R. D. Structure and variation of human ribosomal DNA: molecular analysis of cloned fragments. Gene. 1981 Dec;16(1-3):1–9. doi: 10.1016/0378-1119(81)90055-x. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Galloway R. E., Mets L. J. Atrazine, bromacil, and diuron resistance in chlamydomonas: a single non-mendelian genetic locus controls the structure of the thylakoid binding site. Plant Physiol. 1984 Mar;74(3):469–474. doi: 10.1104/pp.74.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway R. E., Mets L. Non-Mendelian Inheritance of 3-(3,4-Dichlorophenyl)-1,1-dimethylurea-Resistant Thylakoid Membrane Properties in Chlamydomonas. Plant Physiol. 1982 Dec;70(6):1673–1677. doi: 10.1104/pp.70.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Mattoo A. K., Marder J. B., Edelman M., Ellis R. J. General occurrence and structural similarity of the rapidly synthesized, 32,000-dalton protein of the chloroplast membrane. J Biol Chem. 1982 Apr 25;257(8):4583–4587. [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets L. J., Geist L. J. Linkage of a Known Chloroplast Gene Mutation to the Uniparental Genome of CHLAMYDOMONAS REINHARDII. Genetics. 1983 Nov;105(3):559–579. doi: 10.1093/genetics/105.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. M., Grant D. M., Rabert D. K., Harris E. H., Boynton J. E., Gillham N. W. Mutants of Chlamydomonas reinhardtii with physical alterations in their chloroplast DNA. Plasmid. 1982 Mar;7(2):133–151. doi: 10.1016/0147-619x(82)90073-7. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982 Jun;29(2):537–550. doi: 10.1016/0092-8674(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioni R. G., Bennoun P., Chua N. H. A nuclear mutant of Chlamydomonas reinhardtii defective in photosynthetic photophosphorylation. Characterization of the algal coupling factor ATPase. Eur J Biochem. 1981 Jun;117(1):93–102. doi: 10.1111/j.1432-1033.1981.tb06307.x. [DOI] [PubMed] [Google Scholar]
- Renger G. Studies on the structural and functional organization of system II of photosynthesis. The use of trypsin as a structurally selective inhibitor at the outer surface of the thylakoid membrane. Biochim Biophys Acta. 1976 Aug 13;440(2):287–300. doi: 10.1016/0005-2728(76)90063-3. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction endonuclease map of the chloroplast DNA of Chlamydomonas reinhardii. J Mol Biol. 1978 Dec 25;126(4):597–617. doi: 10.1016/0022-2836(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction fragments from Chlamydomonas chloroplast DNA. Methods Enzymol. 1980;65(1):785–795. doi: 10.1016/s0076-6879(80)65073-3. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic analysis of chloroplast DNA in Chlamydomonas. Adv Genet. 1977;19:287–340. doi: 10.1016/s0065-2660(08)60247-3. [DOI] [PubMed] [Google Scholar]
- Spielmann A., Stutz E. Nucleotide sequence of soybean chloroplast DNA regions which contain the psb A and trn H genes and cover the ends of the large single copy region and one end of the inverted repeats. Nucleic Acids Res. 1983 Oct 25;11(20):7157–7167. doi: 10.1093/nar/11.20.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Tischer W., Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim Biophys Acta. 1977 Apr 11;460(1):113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]