Abstract
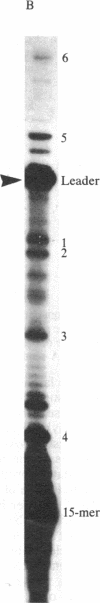
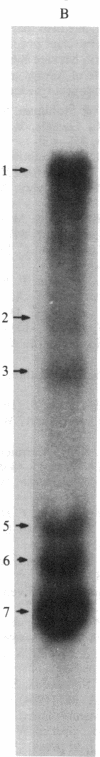
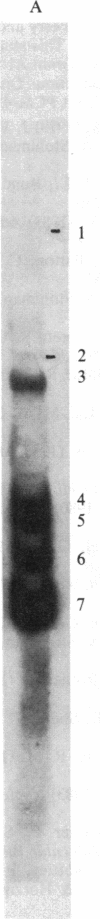
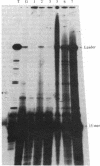
Mouse hepatitis virus, which replicates in cytoplasm, contains leader RNA sequences at the 5' end of the virus-specific mRNAs. We have sequenced this leader RNA by synthesizing cDNA from a synthetic oligodeoxyribonucleotide primer (15-mer) that is complementary to the sequences at the junction site between the leader and body sequences of the mRNAs. The leader sequences on each mRNA have exactly the same size, which span approximately equal to 70 nucleotides. Leader cDNA fragments obtained from several mRNA species were sequenced and found to be identical. Computer analysis of the leader RNA sequences shows that they share extensive sequence homology with the long-terminal-repeat region of several mammalian sarcoma viruses, suggesting possible common functions. This is a novel case of spliced leader sequences in the mRNAs of a cytoplasmic virus. An identical leader sequence is also present at the 5' end of the virion genomic RNA. The leader RNA is thus probably encoded by the virion genomic RNA template and is fused to the different body sequences of the various mRNAs. Since conventional RNA splicing is not involved, a novel mechanism for fusing two noncontiguous RNA segments in the cytoplasm must be utilized during viral transcription. Several minor cDNA bands longer than the leader were also synthesized, suggesting the possible presence of partially homologous sequences in other parts of the genome RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Smeekens S., Rottier P. Sequence of the nucleocapsid gene from murine coronavirus MHV-A59. Nucleic Acids Res. 1983 Feb 11;11(3):883–891. doi: 10.1093/nar/11.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baric R. S., Stohlman S. A., Lai M. M. Characterization of replicative intermediate RNA of mouse hepatitis virus: presence of leader RNA sequences on nascent chains. J Virol. 1983 Dec;48(3):633–640. doi: 10.1128/jvi.48.3.633-640.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton P. R., Ganges R. G., Stohlman S. A. Host cell nuclear function and murine hepatitis virus replication. J Gen Virol. 1981 Oct;56(Pt 2):457–460. doi: 10.1099/0022-1317-56-2-457. [DOI] [PubMed] [Google Scholar]
- Brayton P. R., Lai M. M., Patton C. D., Stohlman S. A. Characterization of two RNA polymerase activities induced by mouse hepatitis virus. J Virol. 1982 Jun;42(3):847–853. doi: 10.1128/jvi.42.3.847-853.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T. Rapid nucleotide sequencing of DNA. Am J Hum Genet. 1979 Jan;31(1):19–28. [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Even J., Sherr C. J., Galibert F. Nucleotide sequences of feline sarcoma virus long terminal repeats and 5' leaders show extensive homology to those of other mammalian retroviruses. J Virol. 1983 Jan;45(1):466–472. doi: 10.1128/jvi.45.1.466-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Brayton P. R., Armen R. C., Patton C. D., Pugh C., Stohlman S. A. Mouse hepatitis virus A59: mRNA structure and genetic localization of the sequence divergence from hepatotropic strain MHV-3. J Virol. 1981 Sep;39(3):823–834. doi: 10.1128/jvi.39.3.823-834.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Patton C. D., Baric R. S., Stohlman S. A. Presence of leader sequences in the mRNA of mouse hepatitis virus. J Virol. 1983 Jun;46(3):1027–1033. doi: 10.1128/jvi.46.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Patton C. D., Stohlman S. A. Further characterization of mRNA's of mouse hepatitis virus: presence of common 5'-end nucleotides. J Virol. 1982 Feb;41(2):557–565. doi: 10.1128/jvi.41.2.557-565.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Patton C. D., Stohlman S. A. Replication of mouse hepatitis virus: negative-stranded RNA and replicative form RNA are of genome length. J Virol. 1982 Nov;44(2):487–492. doi: 10.1128/jvi.44.2.487-492.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Stohlman S. A. RNA of mouse hepatitis virus. J Virol. 1978 May;26(2):236–242. doi: 10.1128/jvi.26.2.236-242.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Leibowitz J. L., Wilhelmsen K. C., Bond C. W. The virus-specific intracellular RNA species of two murine coronaviruses: MHV-a59 and MHV-JHM. Virology. 1981 Oct 15;114(1):39–51. doi: 10.1016/0042-6822(81)90250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. A micromethod for detailed characterization of high molecular weight RNA. Methods Enzymol. 1980;65(1):680–687. doi: 10.1016/s0076-6879(80)65066-6. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Siddell S. G. Coronavirus JHM: nucleotide sequence of the mRNA that encodes nucleocapsid protein. Nucleic Acids Res. 1983 Aug 11;11(15):5045–5054. doi: 10.1093/nar/11.15.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Leibowitz J. L., Bond C. W., Robb J. A. The replication of murine coronaviruses in enucleated cells. Virology. 1981 Apr 15;110(1):225–230. doi: 10.1016/0042-6822(81)90027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]