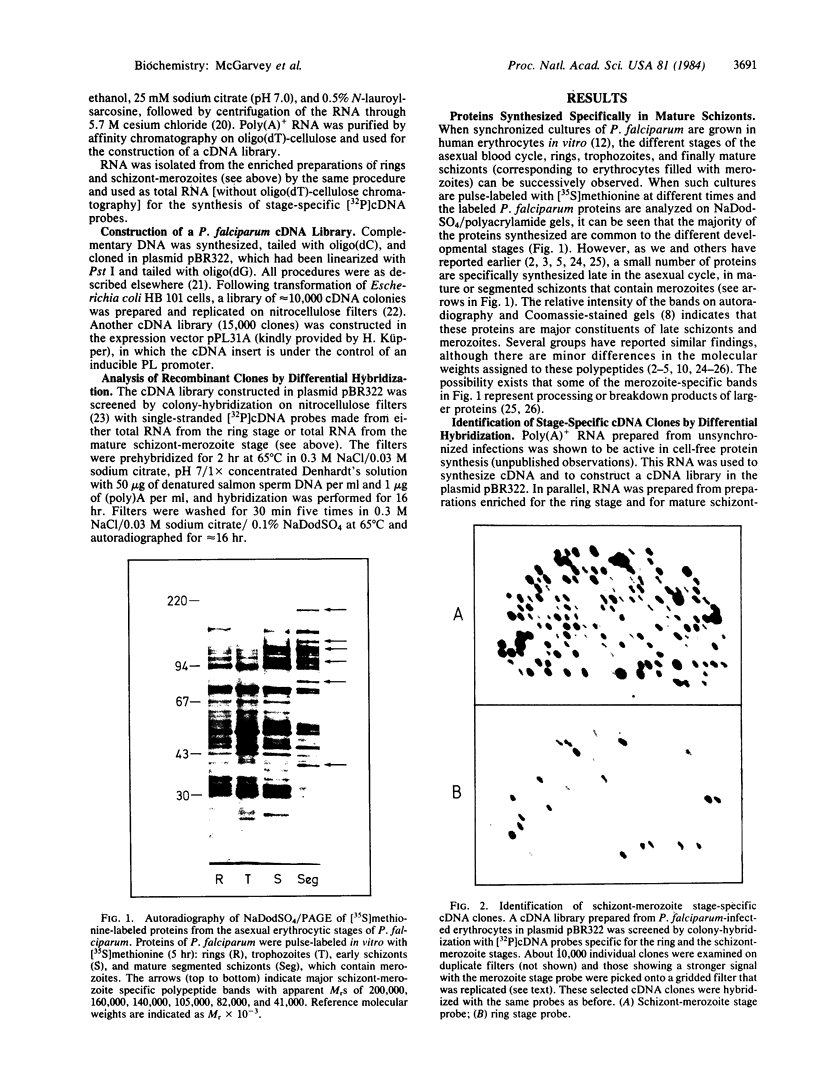
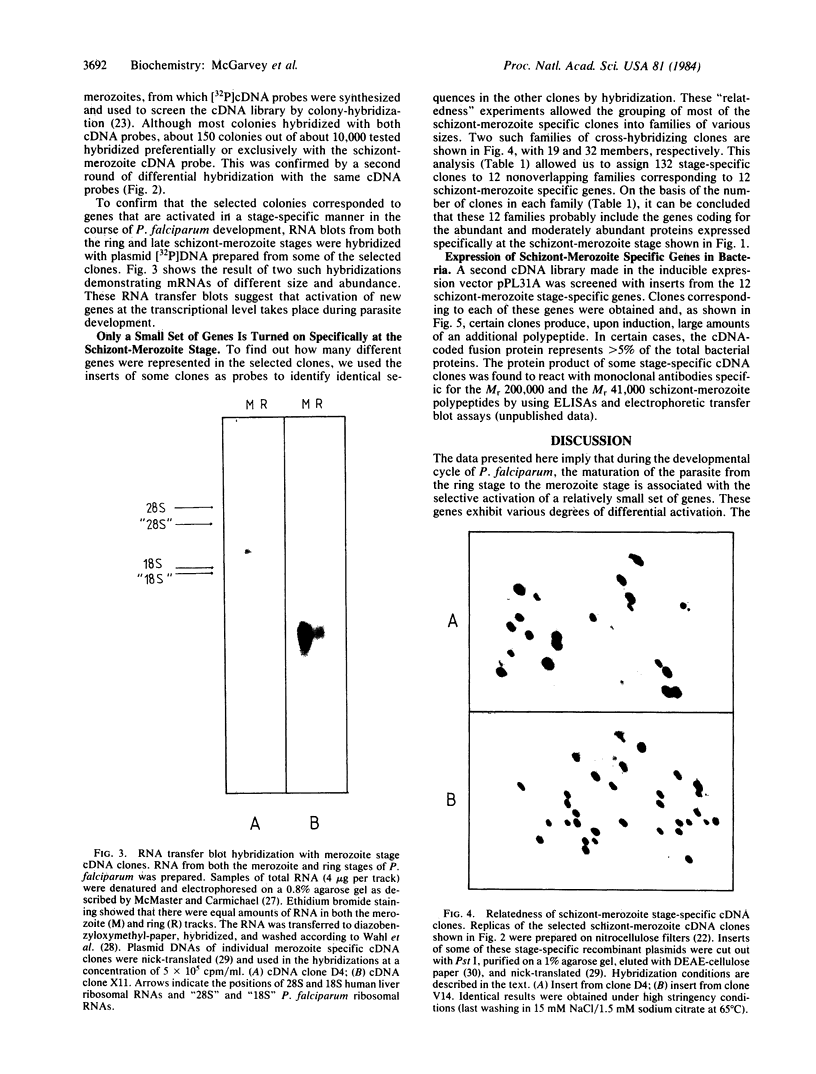
Abstract
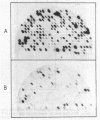
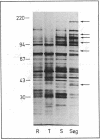
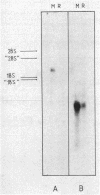
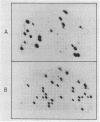
The key steps in the development of a malaria vaccine through gene cloning are the identification of the proteins involved in host protective immunity and the cloning, identification, and expression of the genes coding for these proteins. Recent data have indicated that certain proteins synthesized at the late schizont-merozoite stage of Plasmodium falciparum play a major role in malaria immunity. This paper reports the identification, in a cDNA library, of recombinant clones corresponding to genes expressed specifically during the late schizont-merozoite stage of P. falciparum development. The 132 cDNA clones thus identified out of 10,000 were found to correspond to only 12 different genes, probably representing most of the major schizont-merozoite specific genes. The stage-specific cDNAs can be efficiently expressed in Escherichia coli cells. The protein products of some of these clones are recognized by monoclonal antibodies specific for late schizont-merozoite proteins. We conclude that only a small set of genes is specifically induced in the schizont-merozoite stage and that the stage-specific cDNA clones we have isolated are very likely to include the genes coding for the immunologically relevant proteins of P. falciparum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown G. V., Anders R. F., Mitchell G. F., Heywood P. F. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. Nature. 1982 Jun 17;297(5867):591–593. doi: 10.1038/297591a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Lingelbach K. R., Brown G. V., Saint R. B., Kemp D. J., Anders R. F. Isolate-specific S-antigen of Plasmodium falciparum contains a repeated sequence of eleven amino acids. Nature. 1983 Dec 22;306(5945):751–756. doi: 10.1038/306751a0. [DOI] [PubMed] [Google Scholar]
- Deans J. A., Cohen S. Immunology of malaria. Annu Rev Microbiol. 1983;37:25–49. doi: 10.1146/annurev.mi.37.100183.000325. [DOI] [PubMed] [Google Scholar]
- Deans J. A., Thomas A. W., Inge P. M., Cohen S. Stage-specific protein synthesis by asexual blood stage parasites of Plasmodium falciparum. Mol Biochem Parasitol. 1983 May;8(1):45–51. doi: 10.1016/0166-6851(83)90033-6. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Kafatos F. C. A walk in the chorion locus of Bombyx mori. Cell. 1982 Jun;29(2):633–643. doi: 10.1016/0092-8674(82)90179-9. [DOI] [PubMed] [Google Scholar]
- Ellis J., Ozaki L. S., Gwadz R. W., Cochrane A. H., Nussenzweig V., Nussenzweig R. S., Godson G. N. Cloning and expression in E. coli of the malarial sporozoite surface antigen gene from Plasmodium knowlesi. Nature. 1983 Apr 7;302(5908):536–538. doi: 10.1038/302536a0. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G., Strych W., Mrema J. E. Identification of surface and internal antigens from spontaneously released Plasmodium falciparum merozoites by radio-iodination and metabolic labelling. Z Parasitenkd. 1983;69(6):715–725. doi: 10.1007/BF00927421. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Jungery M., Boyle D., Patel T., Pasvol G., Weatherall D. J. Lectin-like polypeptides of P. falciparum bind to red cell sialoglycoproteins. Nature. 1983 Feb 24;301(5902):704–705. doi: 10.1038/301704a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Cowman A. F., Saint R. B., Brown G. V., Anders R. F. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: detection with antibodies from immune humans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3787–3791. doi: 10.1073/pnas.80.12.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H., Keller W., Kurz C., Forss S., Schaller H., Franze R., Strohmaier K., Marquardt O., Zaslavsky V. G., Hofschneider P. H. Cloning of cDNA of major antigen of foot and mouth disease virus and expression in E. coli. Nature. 1981 Feb 12;289(5798):555–559. doi: 10.1038/289555a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Chung S., Zuker C., Lodish H. F. Selection and analysis of cloned developmentally-regulated Dictyostelium discoideum genes by hybridization-competition. Nucleic Acids Res. 1981 Feb 25;9(4):947–963. doi: 10.1093/nar/9.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Odink K. G., Nicholls S. C., Lockyer M. J., Hillman Y. Cloning of malarial genes coding for high molecular weight antigens: isolation of fragments of Plasmodium falciparum genes coding for proteins of 145 000 molecular weight. Mol Biochem Parasitol. 1984 Jan;10(1):55–66. doi: 10.1016/0166-6851(84)90018-5. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R. Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev. 1982;61:245–269. doi: 10.1111/j.1600-065x.1982.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Ramirez E., Lambert P. H., Miescher P. A. Inhibition of P. falciparum growth in human erythrocytes by monoclonal antibodies. Nature. 1981 Jan 22;289(5795):301–303. doi: 10.1038/289301a0. [DOI] [PubMed] [Google Scholar]
- Reese R. T., Trager W., Jensen J. B., Miller D. A., Tantravahi R. Immunization against malaria with antigen from Plasmodium falciparum cultivated in vitro. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5665–5668. doi: 10.1073/pnas.75.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez da Silva L., Loche M., Dayal R., Perrin L. H. Plasmodium falciparum polypeptides released during in vitro cultivation. Bull World Health Organ. 1983;61(1):105–112. [PMC free article] [PubMed] [Google Scholar]
- Saul A., Myler P., Schofield L., Kidson C. A high molecular weight antigen in Plasmodium falciparum recognized by inhibitory monoclonal antibodies. Parasite Immunol. 1984 Jan;6(1):39–50. doi: 10.1111/j.1365-3024.1984.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Lightholder J., Monroe M. T. Protective Plasmodium knowlesi Mr 74,000 antigen in membranes of schizont-infected rhesus erythrocytes. J Exp Med. 1983 Jul 1;158(1):146–158. doi: 10.1084/jem.158.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A. An effective immunization of experimental monkeys against a human malaria parasite, Plasmodium falciparum. Science. 1977 Jul 22;197(4301):388–389. doi: 10.1126/science.406671. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W., Lanners H. N., Stanley H. A., Langreth S. G. Immunization of owl monkeys to Plasmodium falciparum with merozoites from cultures of a knobless clone. Parasite Immunol. 1983 May;5(3):225–236. doi: 10.1111/j.1365-3024.1983.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Strubin M., Gross N., Accolla R., Carrel S., Mach B. Isolation of cDNA clones encoding HLA-DR alpha chains. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6979–6983. doi: 10.1073/pnas.79.22.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]