Abstract
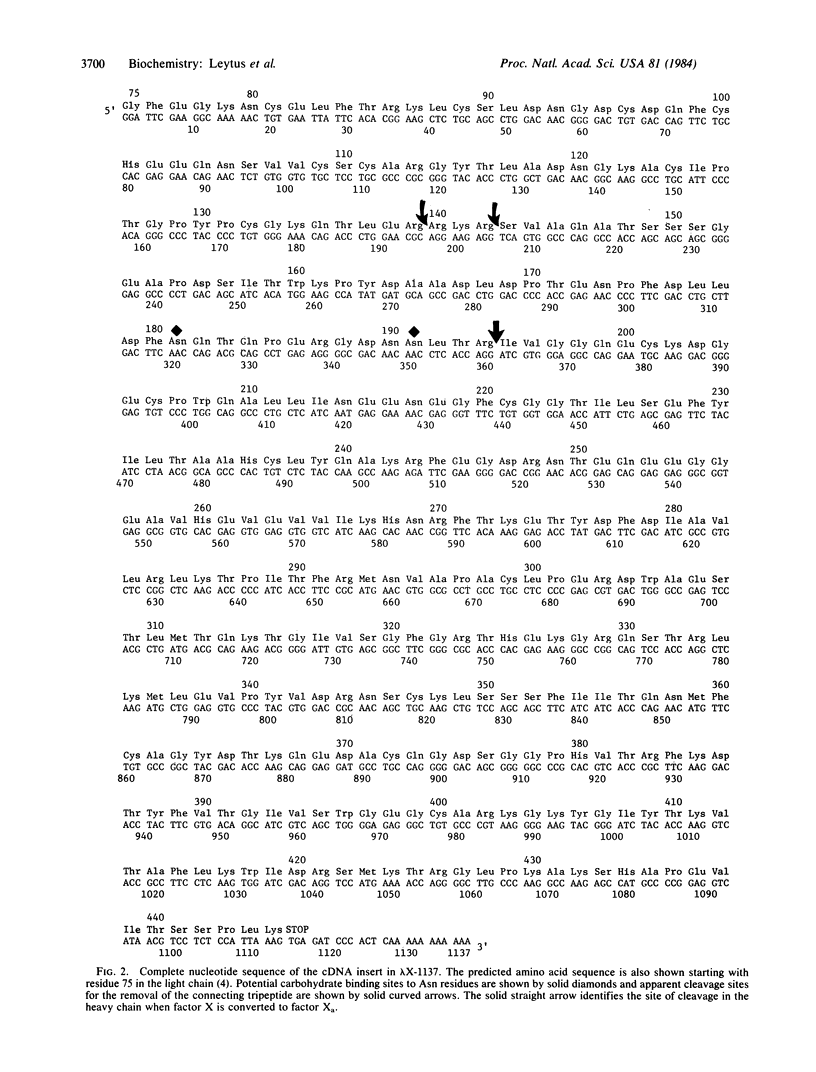
A lambda gt11 cDNA library containing DNA inserts prepared from human liver mRNA has been screened with an antibody to human factor X, a plasma protein participating in the middle phase of the blood coagulation cascade. Ten positive clones were isolated from 2 X 10(6) phage and plaque purified. The cDNA in the phage containing the largest insert has been sequenced and shown to code for human factor X. This cDNA insert contained 1137 base pairs coding for a portion of the light chain of the molecule, a connecting region, the heavy chain, a stop codon, a short 3' noncoding region, and a poly(A) tail. The sequence of A-T-T-A-A-A, which functions as a potential recognition site for polyadenylylation or processing, was present in the 3' end of the coding sequence and preceded the stop codon of TGA by 1 base pair and the poly(A) tail by 14 base pairs. The amino acid sequence deduced from the cDNA indicated that factor X is synthesized as a single-chain polypeptide containing the light and heavy chains connected by an Arg-Lys-Arg tripeptide. The single-chain molecule is then converted to the light and heavy chains by cleavage of two (or more) internal peptide bonds. In plasma, these two chains are linked together by a disulfide bond. The DNA sequence coding for the active site of human factor X showed a high degree of identity with prothrombin and factor IX, two other vitamin K-dependent serine proteases that participate in blood coagulation. These data along with the protein sequence data previously published for the light chain of human factor X establish the complete amino acid sequence for the mature protein present in plasma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bucher D., Nebelin E., Thomsen J., Stenflo J. Identification of gamma-carboxyglutamic acid residues in bovine factors IX and X, and in a new vitamin K-dependent protein. FEBS Lett. 1976 Oct 1;68(2):293–296. doi: 10.1016/0014-5793(76)80456-5. [DOI] [PubMed] [Google Scholar]
- Canfield W. M., Kisiel W. Evidence of normal functional levels of activated protein C inhibitor in combined Factor V/VIII deficiency disease. J Clin Invest. 1982 Dec;70(6):1260–1272. doi: 10.1172/JCI110725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K., Kurachi K., Kisiel W. The role of serine proteases in the blood coagulation cascade. Adv Enzymol Relat Areas Mol Biol. 1979;48:277–318. doi: 10.1002/9780470122938.ch6. [DOI] [PubMed] [Google Scholar]
- Degen S. J., MacGillivray R. T., Davie E. W. Characterization of the complementary deoxyribonucleic acid and gene coding for human prothrombin. Biochemistry. 1983 Apr 26;22(9):2087–2097. doi: 10.1021/bi00278a008. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Davie E. W. Activation of human factor X (Stuart factor) by a protease from Russell's viper venom. Biochemistry. 1977 Nov 29;16(24):5253–5260. doi: 10.1021/bi00643a015. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- DiScipio R. G., Davie E. W. Characterization of protein S, a gamma-carboxyglutamic acid containing protein from bovine and human plasma. Biochemistry. 1979 Mar 6;18(5):899–904. doi: 10.1021/bi00572a026. [DOI] [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H., Titani K. Bovine factor X1 (Stuart factor). Primary structure of the light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):16–19. doi: 10.1073/pnas.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. The cDNA for the beta-subunit of human chorionic gonadotropin suggests evolution of a gene by readthrough into the 3'-untranslated region. Nature. 1980 Aug 14;286(5774):684–687. doi: 10.1038/286684a0. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Legaz M. E., Davie E. W. The mechanism of activation of bovine factor X (Stuart factor) by intrinsic and extrinsic pathways. Biochemistry. 1974 Dec 17;13(26):5290–5299. doi: 10.1021/bi00723a006. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factor X 1 (Stuart factor). Mechanism of activation by protein from Russell's viper venom. Biochemistry. 1972 Dec 19;11(26):4892–4899. doi: 10.1021/bi00776a003. [DOI] [PubMed] [Google Scholar]
- Graves C. B., Munns T. W., Willingham A. K., Strauss A. W. Rat factor X is synthesized as a single chain precursor inducible by prothrombin fragments. J Biol Chem. 1982 Nov 10;257(21):13108–13113. [PubMed] [Google Scholar]
- Jesty J., Nemerson Y. Purification of Factor VII from bovine plasma. Reaction with tissue factor and activation of Factor X. J Biol Chem. 1974 Jan 25;249(2):509–515. [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W., Sasagawa T., Howald W. N., Kwa E. Y., Weinstein B. Complete amino acid sequence of the light chain of human blood coagulation factor X: evidence for identification of residue 63 as beta-hydroxyaspartic acid. Biochemistry. 1983 Jun 7;22(12):2875–2884. doi: 10.1021/bi00281a016. [DOI] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W. The occurrence of beta-hydroxyaspartic acid in the vitamin K-dependent blood coagulation zymogens. Biochem Biophys Res Commun. 1983 Aug 30;115(1):8–14. doi: 10.1016/0006-291x(83)90961-0. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Yamashita K., Fujikawa K., Titani K., Kobata A. The structures of the carbohydrate moieties of bovine blood coagulation factor X. J Biol Chem. 1980 Apr 25;255(8):3526–3531. [PubMed] [Google Scholar]
- Pepper D. S., Prowse C. Chromatography of human prothrombin complex or dextran sulphate agarose. Thromb Res. 1977 Nov;11(5):687–692. doi: 10.1016/0049-3848(77)90026-3. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugo T., Fernlund P., Stenflo J. erythro-beta-Hydroxyaspartic acid in bovine factor IX and factor X. FEBS Lett. 1984 Jan 2;165(1):102–106. doi: 10.1016/0014-5793(84)80023-x. [DOI] [PubMed] [Google Scholar]
- Thøgersen H. C., Petersen T. E., Sottrup-Jensen L., Magnusson S., Morris H. R. The N-terminal sequences of blood coagulation factor X1 and X2 light chains. Mass-spectrometric identification of twelve residues of gamma-carboxyglutamic acid in their vitamin K-dependent domains. Biochem J. 1978 Nov 1;175(2):613–627. doi: 10.1042/bj1750613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Fujikawa K., Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H. Bovine factor X1 (Stuart factor): amino-acid sequence of heavey chain. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3082–3086. doi: 10.1073/pnas.72.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]



