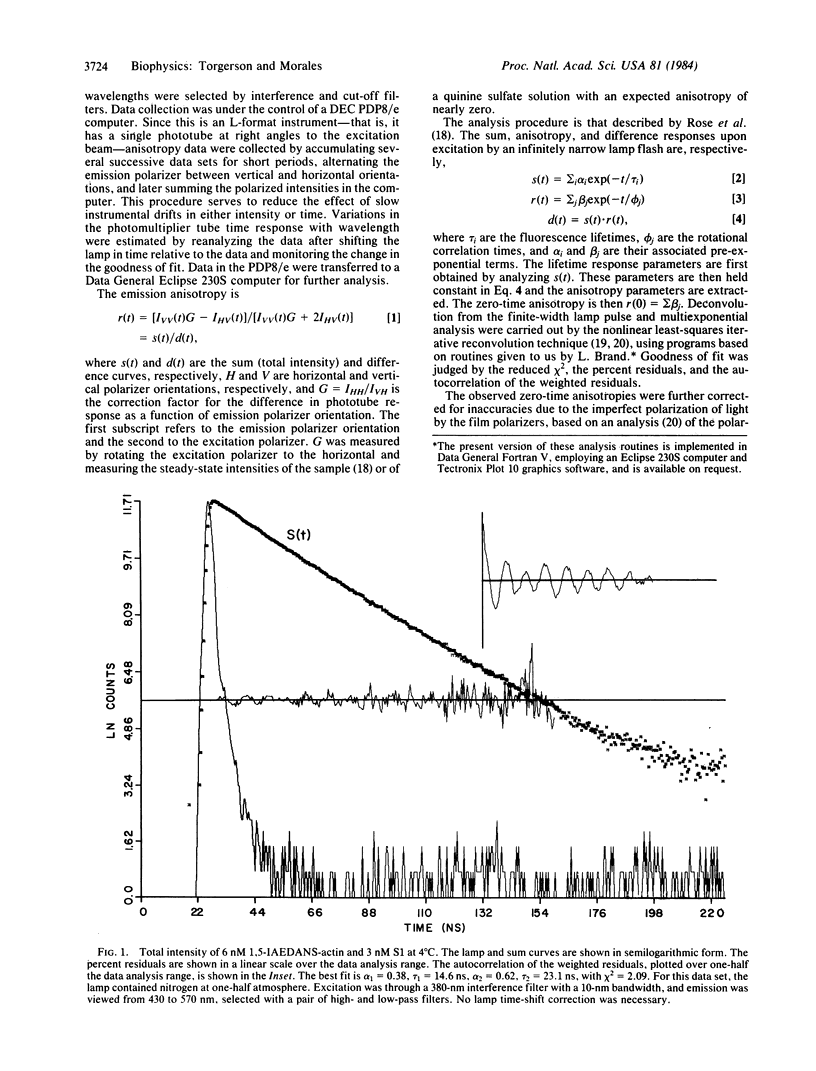
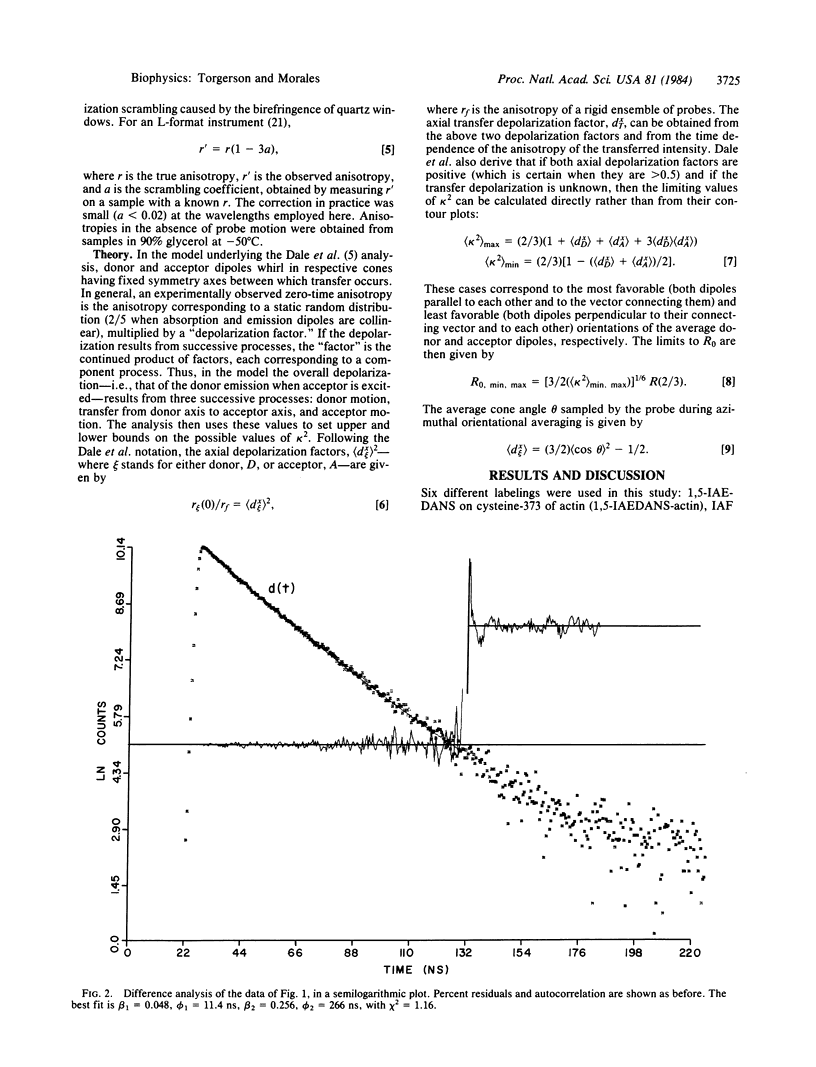
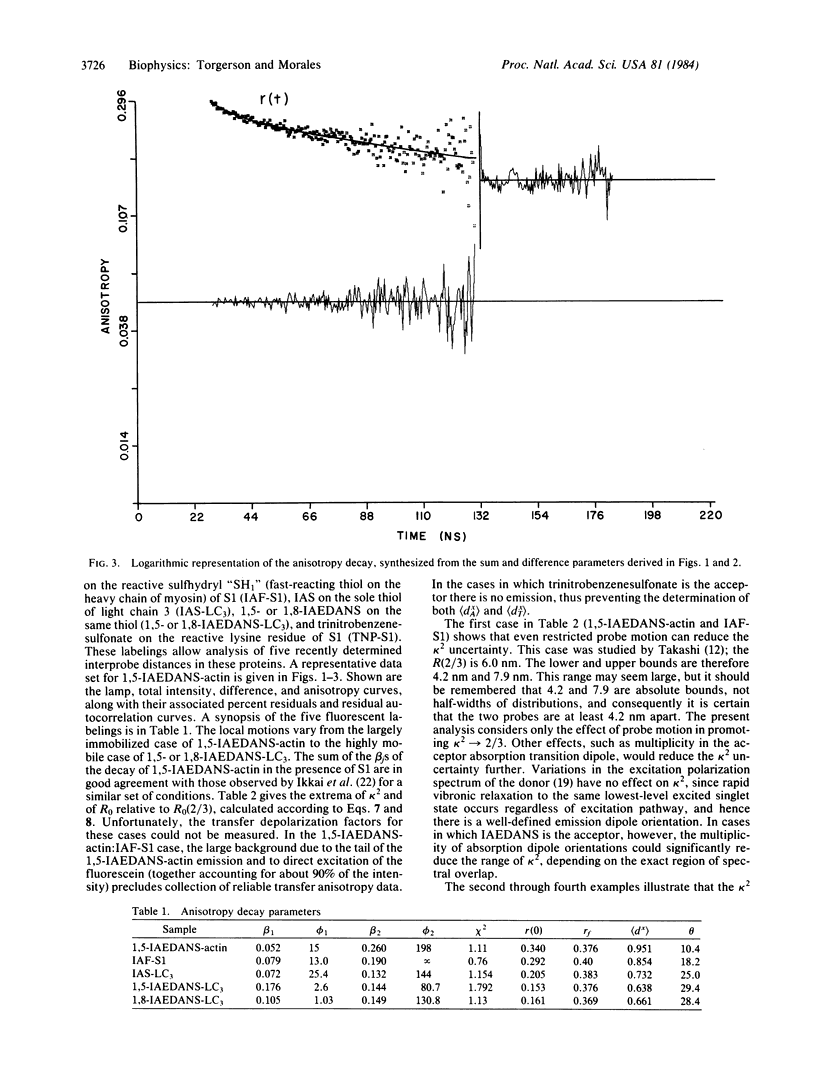
Abstract
Fluorescence anisotropy decays were used to quantify the degree of rapid librational motion associated with several fluorescent probes attached to contractile proteins. This information allows an analysis of how far kappa 2, the resonance energy transfer orientation factor, may deviate from the dynamically averaged value of 2/3. Extrema can then be set on R0, the critical transfer distance, and hence on the interprobe distance. These results set maximum ranges on possible distances between several probe pairs used in the mapping of contractile protein structure by resonance energy transfer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badea M. G., Brand L. Time-resolved fluorescence measurements. Methods Enzymol. 1979;61:378–425. doi: 10.1016/0076-6879(79)61019-4. [DOI] [PubMed] [Google Scholar]
- Botts J., Takashi R., Torgerson P., Hozumi T., Muhlrad A., Mornet D., Morales M. F. On the mechanism of energy transduction in myosin subfragment 1. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2060–2064. doi: 10.1073/pnas.81.7.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon J. W., Hammes G. G. Investigation of reduced nicotinamide adenine dinucleotide phosphate and acyl-binding sites on avian fatty acid synthase. Biochemistry. 1982 Jun 8;21(12):2863–2870. doi: 10.1021/bi00541a009. [DOI] [PubMed] [Google Scholar]
- Cheung H. C., Wang C. K., Garland F. Fluorescence energy transfer studies of skeletal troponin C proximity between methionine-25 and cysteine-98. Biochemistry. 1982 Oct 12;21(21):5135–5142. doi: 10.1021/bi00264a005. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R. The use of singlet-singlet energy transfer to study macromolecular assemblies. Methods Enzymol. 1978;48:347–379. doi: 10.1016/s0076-6879(78)48019-x. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem. 1974 Jun;59(2):583–598. doi: 10.1016/0003-2697(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Haas E., Katchalski-Katzir E., Steinberg I. Z. Effect of the orientation of donor and acceptor on the probability of energy transfer involving electronic transitions of mixed polarization. Biochemistry. 1978 Nov 14;17(23):5064–5070. doi: 10.1021/bi00616a032. [DOI] [PubMed] [Google Scholar]
- Houk T. W., Jr, Ue K. The measurement of actin concentration in solution: a comparison of methods. Anal Biochem. 1974 Nov;62(1):66–74. doi: 10.1016/0003-2697(74)90367-4. [DOI] [PubMed] [Google Scholar]
- Hudson E. N., Weber G. Synthesis and characterization of two fluorescent sulfhydryl reagents. Biochemistry. 1973 Oct 9;12(21):4154–4161. doi: 10.1021/bi00745a019. [DOI] [PubMed] [Google Scholar]
- Ikkai T., Wahl P., Auchet J. C. Anisotropy decay of labelled actin. Evidence of the flexibility of the peptide chain in F-actin molecules. Eur J Biochem. 1979 Jan 15;93(2):397–408. doi: 10.1111/j.1432-1033.1979.tb12836.x. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Lowey S. Fluorescence energey transfer in myosin subfragment-1. Biochemistry. 1980 Feb 19;19(4):774–784. doi: 10.1021/bi00545a025. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Putnam S., Morales M. Time-dependent fluorescence depolarization and lifetime studies of myosin subfragment-one in the presence of nucleotide and actin. J Supramol Struct. 1975;3(2):162–168. doi: 10.1002/jss.400030209. [DOI] [PubMed] [Google Scholar]
- Ross J. B., Schmidt C. J., Brand L. Time-resolved fluorescence of the two tryptophans in horse liver alcohol dehydrogenase. Biochemistry. 1981 Jul 21;20(15):4369–4377. doi: 10.1021/bi00518a021. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Takashi R. Fluorescence energy transfer between subfragment-1 and actin points in the rigor complex of actosubfragment-1. Biochemistry. 1979 Nov 13;18(23):5164–5169. doi: 10.1021/bi00590a021. [DOI] [PubMed] [Google Scholar]
- Takashi R., Muhlrad A., Botts J. Spatial relationship between a fast-reacting thiol and a reactive lysine residue of myosin subfragment 1. Biochemistry. 1982 Oct 26;21(22):5661–5668. doi: 10.1021/bi00265a042. [DOI] [PubMed] [Google Scholar]
- Tonomura Y., Appel P., Morales M. On the molecular weight of myosin. II. Biochemistry. 1966 Feb;5(2):515–521. doi: 10.1021/bi00866a017. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Weeds A. G. Studies on the role of myosin alkali light chains. Recombination and hybridization of light chains and heavy chains in subfragment-1 preparations. J Mol Biol. 1977 Jan 25;109(3):455–470. doi: 10.1016/s0022-2836(77)80023-5. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]


