Abstract
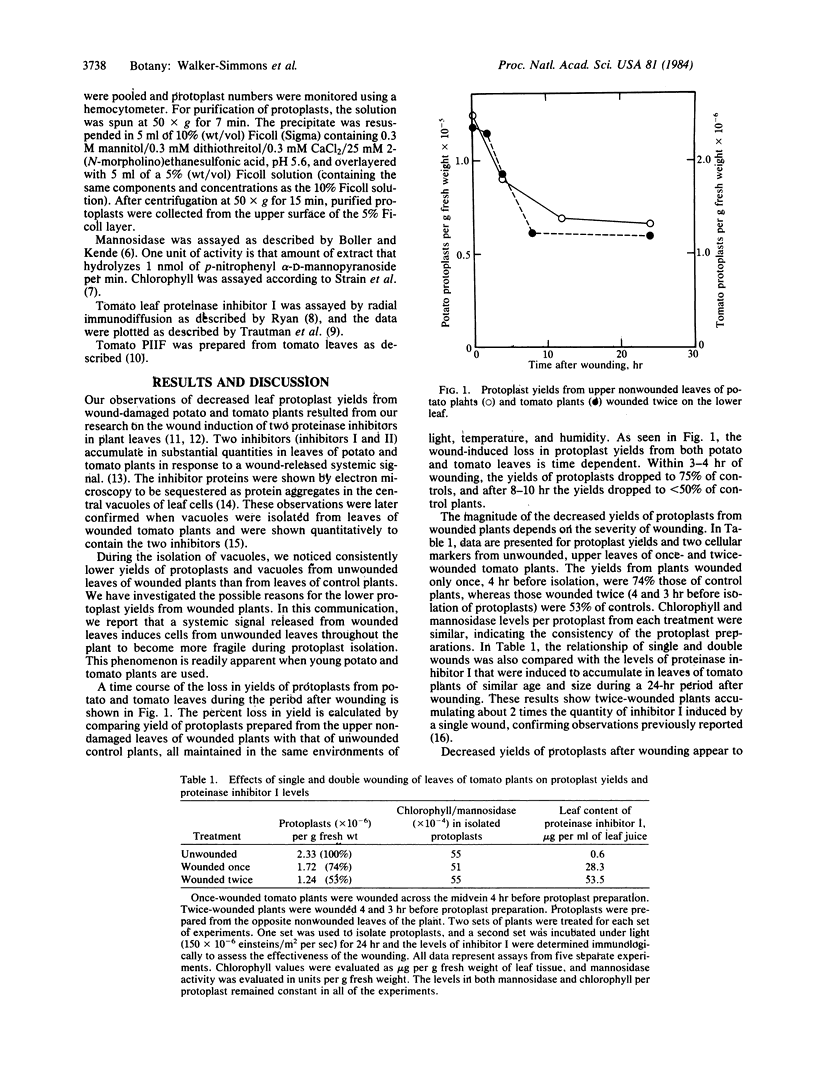
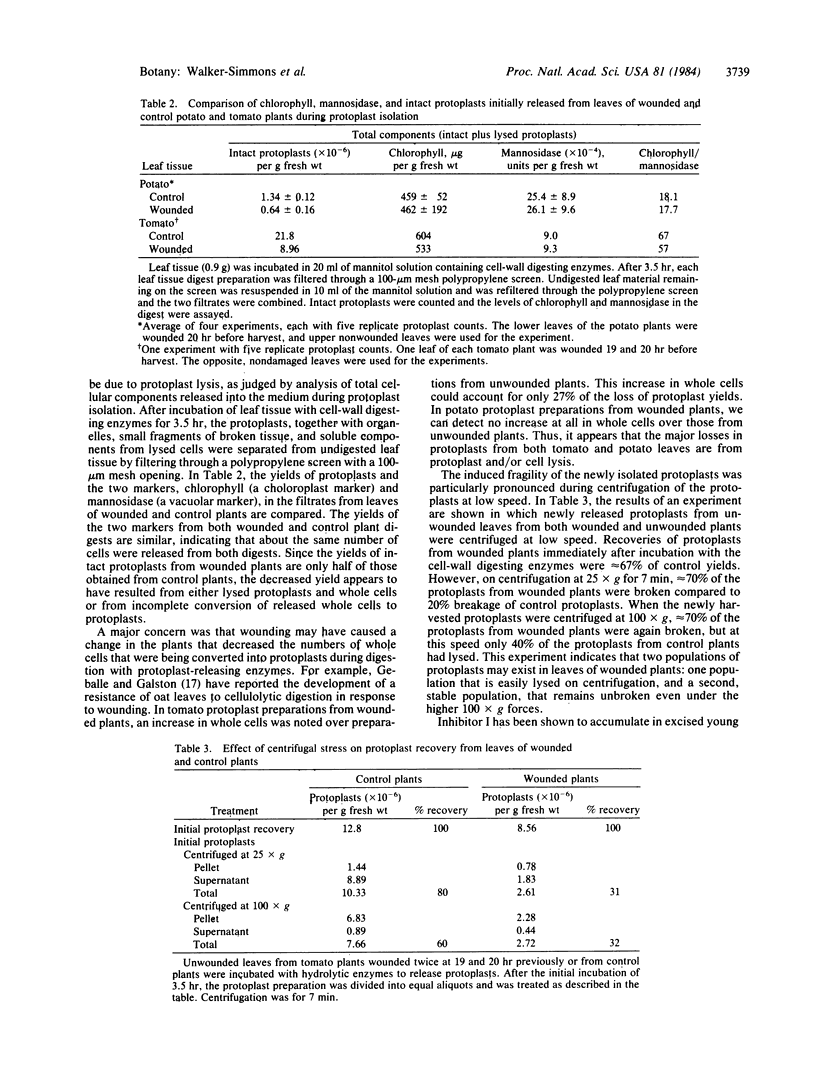
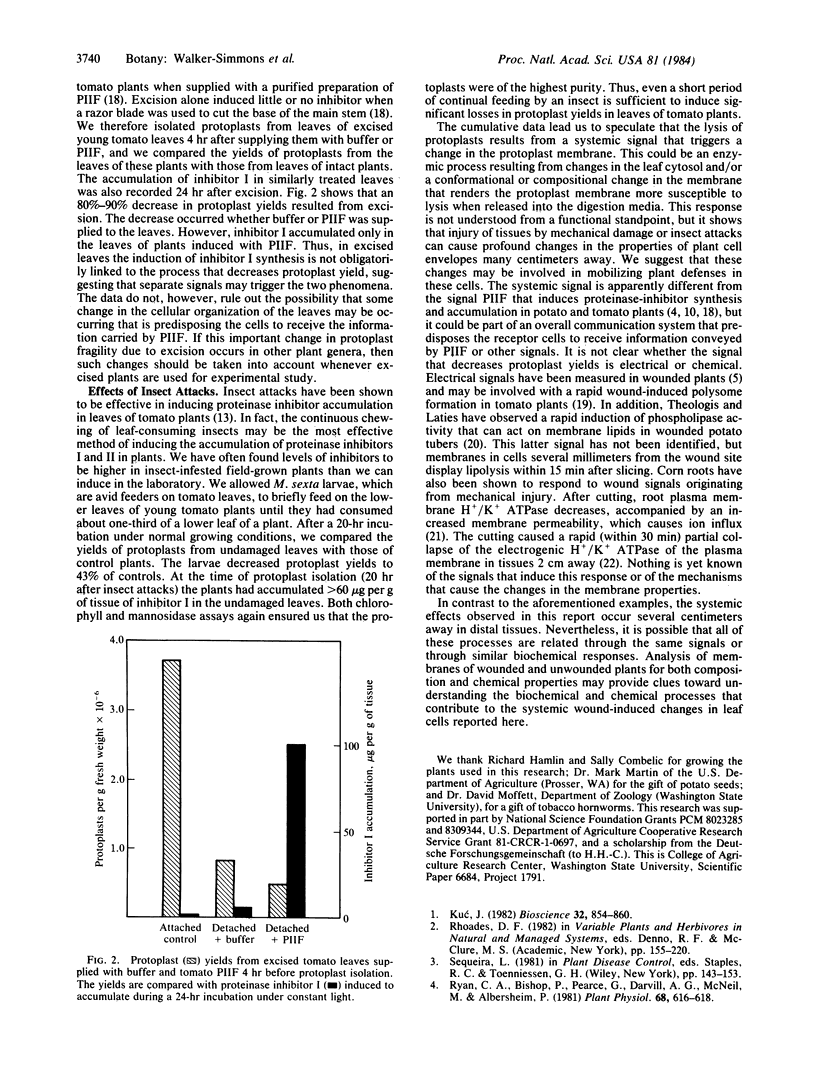
Within 4 hr after wounding the lower leaves of young potato and tomato plants, a rapid and remarkable change is induced in the cells of upper undamaged leaves that results in extensive lysis of protoplasts during their isolation. Protoplast yields from unwounded upper leaves, 4 hr after wounding a lower leaf by crushing with a hemostat, decreased 25% below yields from leaves of unwounded plants. From 8 to >20 hr after wounding, protoplast yields were less than half of those from control plants. Multiple woundings decreased yields even further, as did chewing of the lower leaves by tobacco hornworms over a period of several minutes. In addition, within 4 hr of excising young tomato plants at their base with a razor blade, a 90% decrease in leaf protoplast yields was recorded. The major loss of protoplasts induced by wounding was primarily due to an increased cell lysis during protoplast isolation. Cell lysis was apparently due to a weakened cell membrane, because newly recovered protoplasts released from leaves of wounded plants were extremely fragile and exhibited 70% lysis during low speed centrifugation, compared to 20% lysis of protoplasts recovered from control plants. We conclude that a signal is released by wounding that is rapidly transmitted or transported through the plants to induce a profound change in the leaf cell membranes that renders them fragile during protoplast isolation. It is proposed that this signal may play a role in inducing cellular changes in the plant cells as part of their responses to environmental stress such as pest attacks.
Keywords: pest attacks, protoplasts, proteinase inhibitors, tomato and potato leaves
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. D., Makus D. J., Pearce G., Ryan C. A. Proteinase inhibitor-inducing factor activity in tomato leaves resides in oligosaccharides enzymically released from cell walls. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3536–3540. doi: 10.1073/pnas.78.6.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound signal. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geballe G. T., Galston A. W. Wound-induced resistance to cellulase in oat leaves. Plant Physiol. 1982 Sep;70(3):781–787. doi: 10.1104/pp.70.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Assay and Biochemical Properties of the Proteinase Inhibitor-inducing Factor, a Wound Hormone. Plant Physiol. 1974 Sep;54(3):328–332. doi: 10.1104/pp.54.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A., Bishop P., Pearce G. A sycamore cell wall polysaccharide and a chemically related tomato leaf polysaccharide possess similar proteinase inhibitor-inducing activities. Plant Physiol. 1981 Sep;68(3):616–618. doi: 10.1104/pp.68.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967 Jun;19(3):434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Wound-regulated synthesis and vacuolar compartmentation of proteinase inhibitors in plant leaves. Curr Top Cell Regul. 1980;17:1–23. doi: 10.1016/b978-0-12-152817-1.50005-5. [DOI] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Wound-induced membrane lipid breakdown in potato tuber. Plant Physiol. 1981 Jul;68(1):53–58. doi: 10.1104/pp.68.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautman R., Cowan K. M., Wagner G. G. Data processing for radial immunodiffusion. Immunochemistry. 1971 Oct;8(10):901–916. doi: 10.1016/0019-2791(71)90429-0. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Immunological Identification of Proteinase Inhibitors I and II in Isolated Tomato Leaf Vacuoles. Plant Physiol. 1977 Jul;60(1):61–63. doi: 10.1104/pp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi G., Hanson J. B. Calcium influx into corn roots as a result of cold shock. Plant Physiol. 1982 Jul;70(1):318–319. doi: 10.1104/pp.70.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]



