Abstract
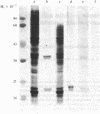
A murine monoclonal anti-DNA antibody ( PME77 ) has been found to bind tightly to the plasma membrane of Raji cells. We show here that this monoclonal anti-DNA antibody reacts in a radioimmunoassay with the cell surface of a variety of mammalian cell types and that the antigenic determinant recognized by the monoclonal anti-DNA antibody at the surface of Raji cells is resistant to DNase. It belongs to polypeptides removed from the cell surface by a mild proteinase K treatment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bresnihan B., Oliver M., Williams B., Hughes G. R. An antineuronal antibody cross-reacting with erythrocytes and lymphocytes in systemic lupus erythematosus. Arthritis Rheum. 1979 Apr;22(4):313–320. doi: 10.1002/art.1780220401. [DOI] [PubMed] [Google Scholar]
- Burke B., Griffiths G., Reggio H., Louvard D., Warren G. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1982;1(12):1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of an integral membrane glycoprotein associated with the microfilaments of pig intestinal microvilli. EMBO J. 1983;2(3):469–475. doi: 10.1002/j.1460-2075.1983.tb01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Lancashire C. L. Comparison of intestinal brush-border 95-Kdalton polypeptide and alpha-actinins. J Cell Biol. 1980 Mar;84(3):655–667. doi: 10.1083/jcb.84.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Jacob L., Tron F. Monoclonal anti-deoxyribonucleic antibodies. I. Isotype and specificity studies. J Immunol. 1982 Feb;128(2):895–898. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes N. F., Tolnai M. E., Silveira N. P., Gilbertsen R. B., Metzgar R. S. Technical aspects of the rosette tests used to detect human complement receptor (B) and sheep erythrocyte-binding (T) lymphocytes. J Immunol. 1973 Sep;111(3):860–867. [PubMed] [Google Scholar]
- Pontes de Carvalho L. C., Templeman J., Wick G., Roitt I. M. The role of self-antigen in the development of autoimmunity in Obese strain chickens with spontaneous autoallergic thyroiditis. J Exp Med. 1982 May 1;155(5):1255–1266. doi: 10.1084/jem.155.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudikoff S., Potter M., Segal D. M., Padlan E. A., Davies D. R. Crystals of phosphorylcholine-binding Fab-fragments from mouse myeloma proteins: preparation and x-ray analysis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3689–3692. doi: 10.1073/pnas.69.12.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles R. P., Messner R. P., Bankhurst A. D. Cross--reactivity of antilymphocyte and antinuclear antibodies in systemic lupus erythematosus. Clin Immunol Immunopathol. 1979 Nov;14(3):292–299. doi: 10.1016/0090-1229(79)90155-7. [DOI] [PubMed] [Google Scholar]
- Talal N., Gallo R. C. Antibodies to a DNA:RNA hybrid in systemic lupus erythematosus measured by a cellulose ester filter radioimmunoassay. Nat New Biol. 1972 Dec 20;240(103):240–242. doi: 10.1038/newbio240240a0. [DOI] [PubMed] [Google Scholar]
- Tron F., Charron D., Bach J. F., Talal N. Establishment and characterization of a murine hybridoma secreting monoclonal anti-DNA autoantibody. J Immunol. 1980 Dec;125(6):2805–2809. [PubMed] [Google Scholar]
- Tron F., Jacob L., Bach J. F. Binding of a murine monoclonal anti-DNA antibody to Raji cells. Implications for the interpretation of the Raji cell assay for immune complexes. Eur J Immunol. 1984 Mar;14(3):283–286. doi: 10.1002/eji.1830140316. [DOI] [PubMed] [Google Scholar]